Coordination Compounds Class 12 Notes

Class 12 Chemistry Chapter 9 Coordination Compounds Notes- Pdf Download
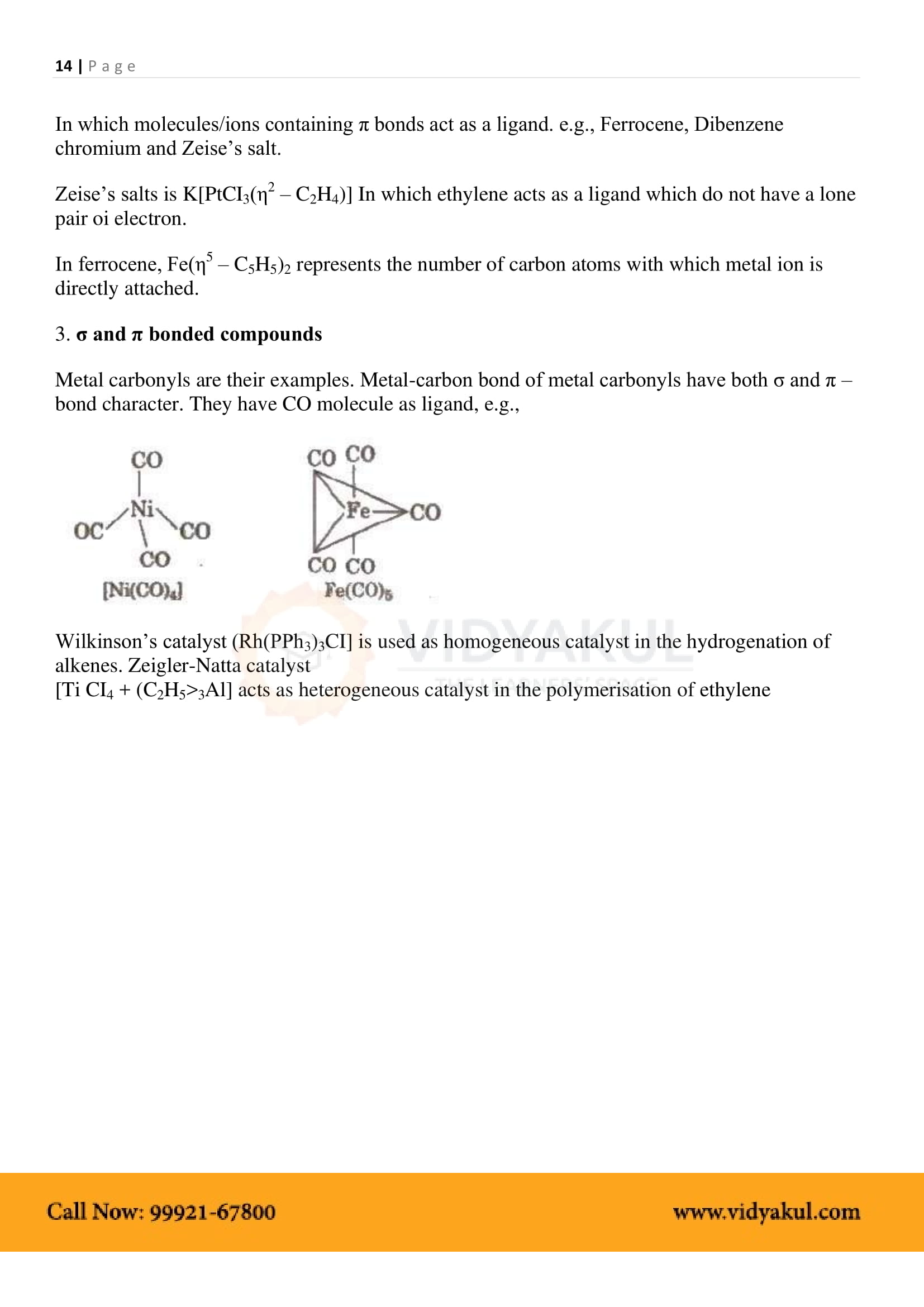
Chapter 9 Coordination Compounds
In this part students can find all the detailed notes for Grade 12 Chemistry chapter 9. Notes crafted by our experts and Step-by-step presentation to help students from CBSE Grade 12. grasp concepts quickly. They are also effective in reinforcing conceptual knowledge of the subject.
CBSE CLASS 12th CHEMISTRY 9 NOTES
Points to Remember
Students can refer to the important points related to Chemistry Class 12 Class 9 mentioned below and note down the points to have a quick revision during final examinations.
Double Salts: These addition compounds are stable only in a solid state but lose their identity in solution form.
Coordination Compounds: These addition compounds retain their identity in a solid state as well as in solution form. In these compounds, a central atom is bonded to a number of groups through coordinate bonds.
Coordination Sphere: The central metal atom/ion along with ligands is written in a square bracket, [ ], called coordination sphere.
Counter Ions: The ions present outside the coordination sphere/entity are called counter ions.
Charge on Complex Ion: It is the algebraic sum of the charges on all the ligands and central atom/ion.
Ligands: An atom or group of atoms that can donate a pair of electrons to the central metal atom. These are of the type monodentate, bidentate, tridentate …… polydentate depending upon the number of donor sites.
Chelating Ligands: Multidentate ligands are chelating ligands if they can be attached to a particular metal atom simultaneously through two or more two sites.
Ambidentate Ligands: Unidentate ligands which have more than one donor atom through which they can co-ordinate to the central atom.
Coordination Number: The total number of ligands attached to the central metal atom in the coordination sphere.
Coordination polyhedron: A 3D spatial arrangement of ligands about the central atom.
Topics and Sub-Topics
Chapter 9, Coordination Compounds, is one of the interesting chapters in chemistry. Students who master the concepts can easily tackle any type of exam question. Although the learning methods may seem confusing at first, persistently practicing the techniques and methods will help students master the material and get the best score. Students should practice as many questions as possible to become proficient in handling easy and difficult question types.
All notes compiled by Vidyakul for grade 12 chapter 9 questions are based on the latest textbooks. Below mentioned are the important topics related to Class 12 Chemistry Chapter 9.
Download this solution for FREE Download This PDF
Download Vidyakul App for more videos, PDF's and Free video lectures.









Important Links:
Few Important Questions
What are ‘Coordination compounds’?
It is a compound that contains coordinate bonds, typically between a central metal atom and a number of other atoms or groups.
What is a ‘Valence bond’?
Valence bond is a chemical bonding theory that explains the chemical bonding between two atoms.
What is an ‘Enantiomer’?
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other.
Practice Questions
Explain the difference between unidentate, bidentate, and ambidentate ligands with two examples each.
What does the stability of a coordination compound in solution mean? What are the factors that govern stability complexes?
Explain Werner’s postulates for the bonding in coordination compounds.
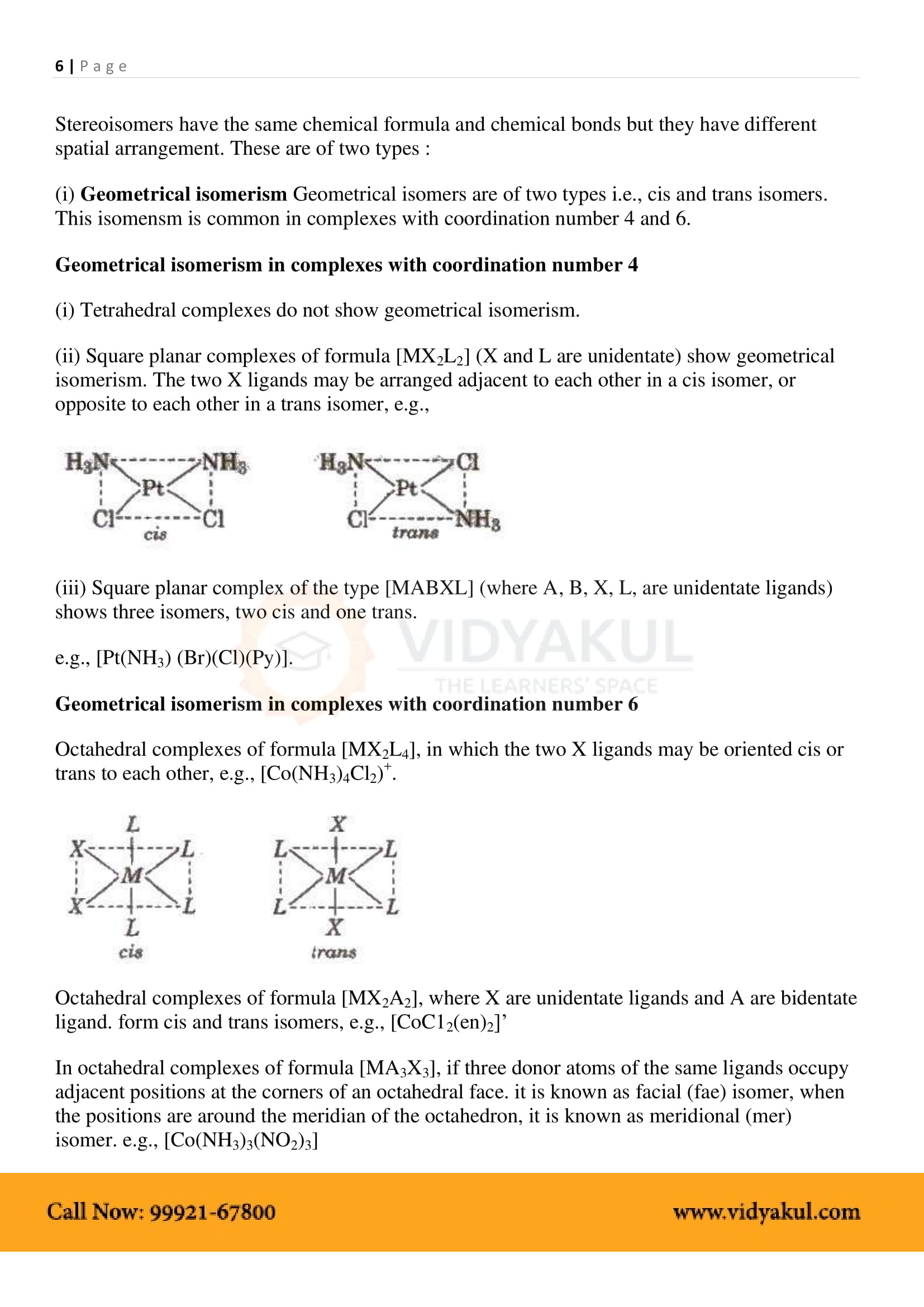
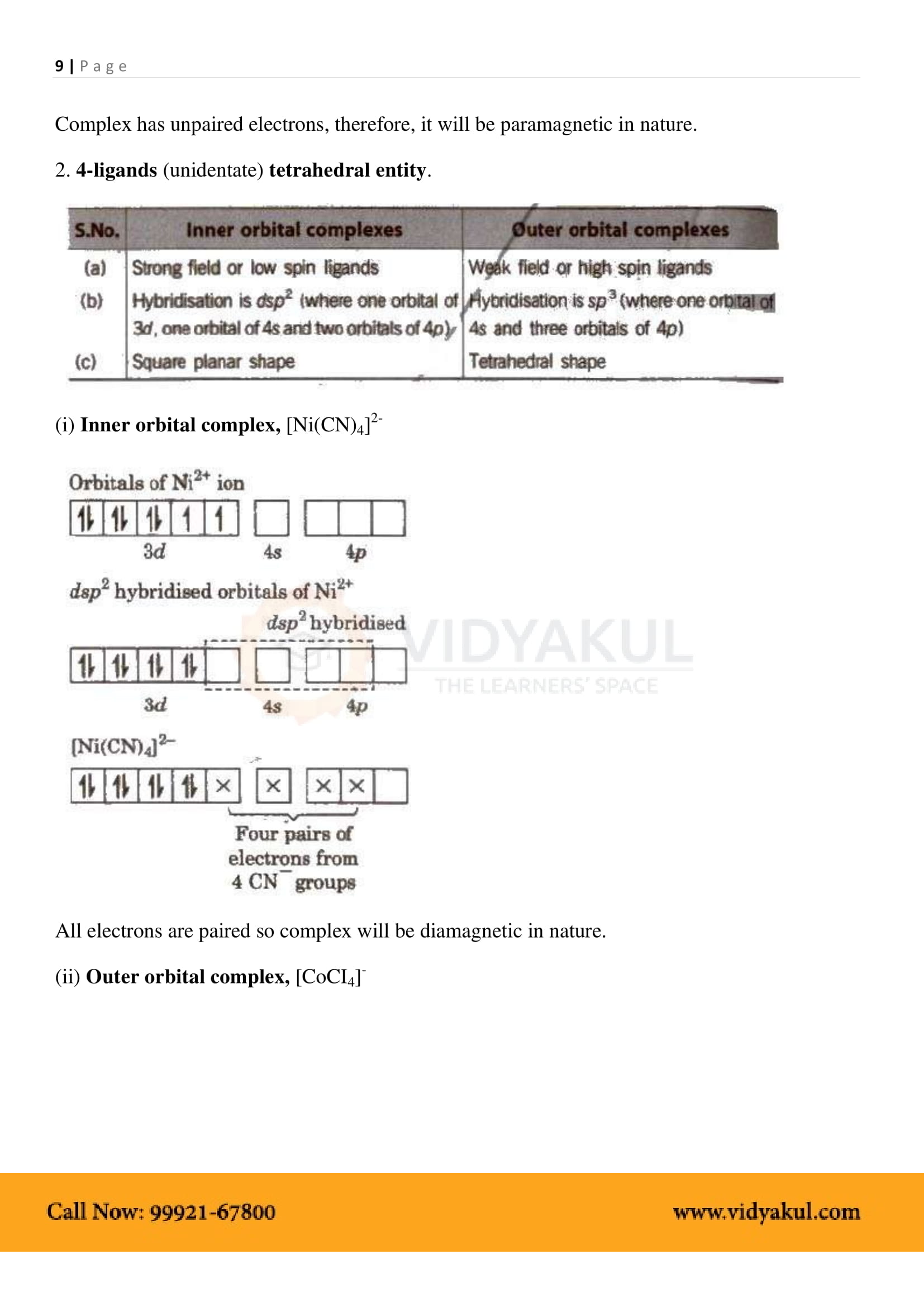
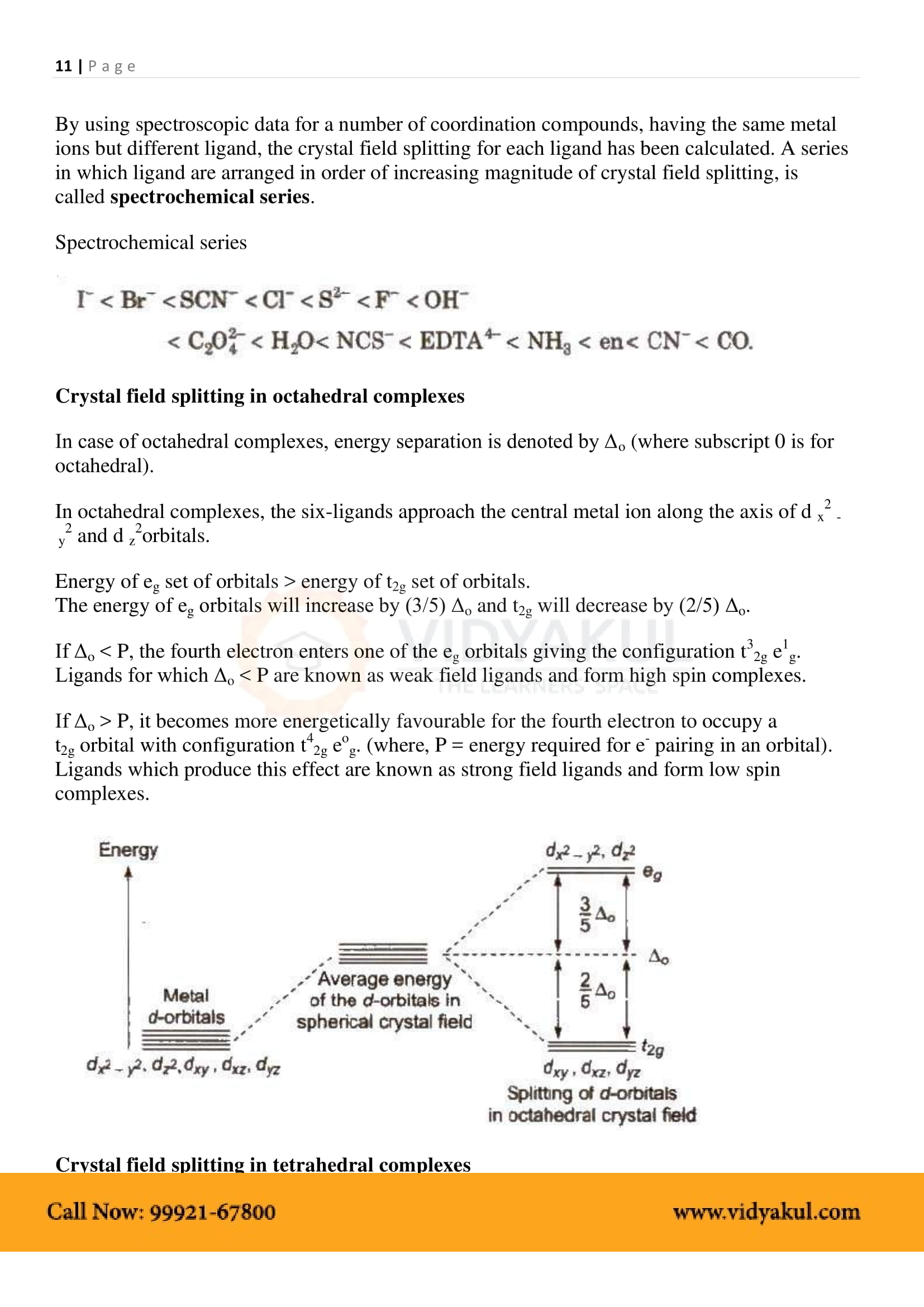
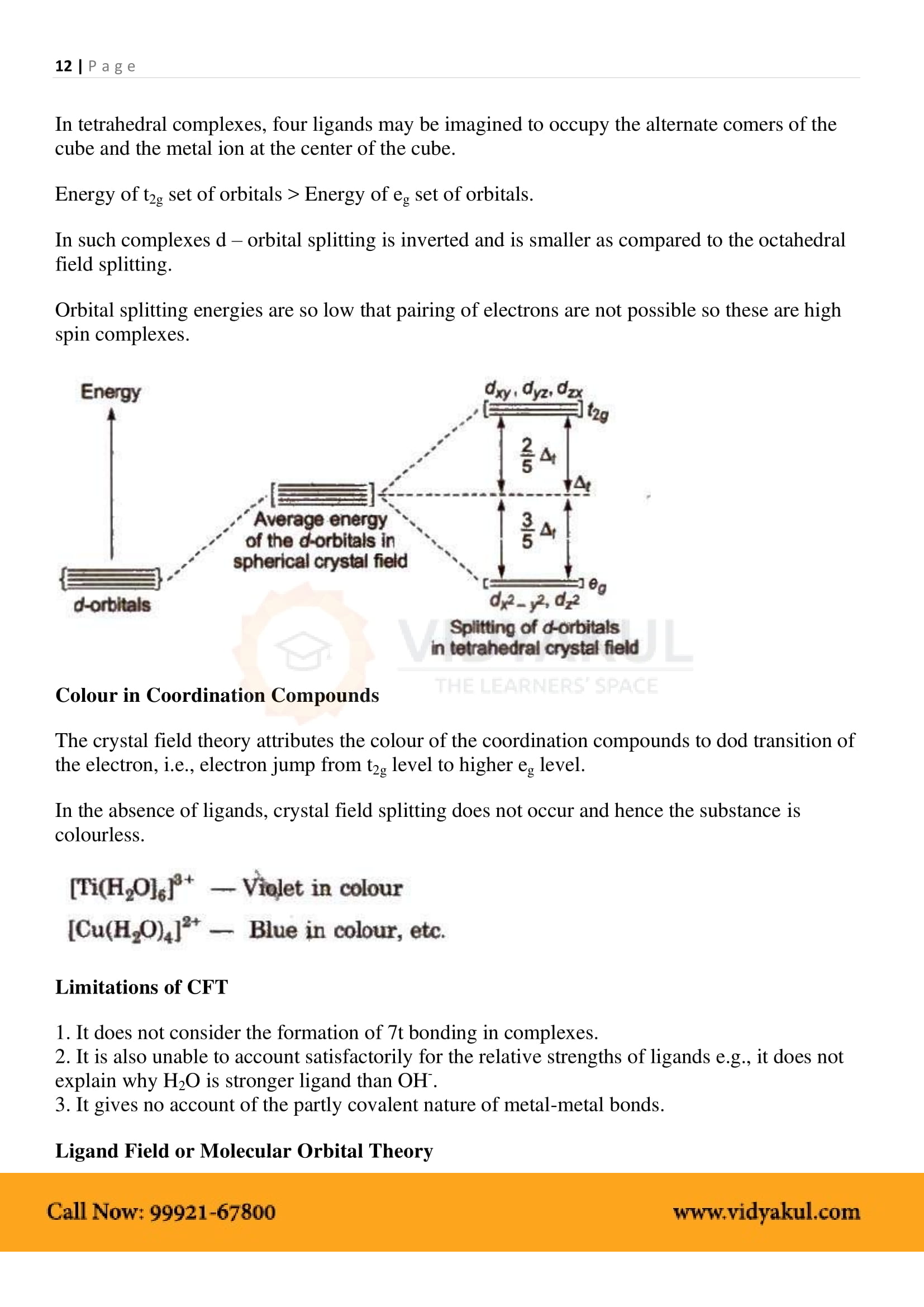
What are the different types of isomerism possible for coordination compounds? Give an example for each.
Explain the chelate effect with an example.
What is a coordination entity? Give one example
How many geometrical isomers are possible for Co(NH3)3Cl3?



