Surface Chemistry Class 12 Notes

Class 12 Chemistry Chapter 5 Surface Chemistry Notes – PDF Download
Chapter 5 Surface Chemistry
Getting good grades in chemistry is not an easy task for many students. However, with step-by-step NCERT notes provided for each chapter question, illustrations, and explanations, students can easily master the Surface Chemistry chapter and master all the concepts in it.
CBSE Class 12 Chapter 5 talks about adsorption, mechanisms, and forms of adsorption, isotherms of adsorption, applications of adsorption, colloids, enzyme catalysis, preparation of colloids, and many more. Vidyakul offers practice questions for all chapter-related sub-topics. Students must practice questions in the text provided by Vidyakul to get a good score. Keep reading to access the NCERT notes for Grade 12 Chemistry Chapter 5.
CBSE CLASS 12th CHEMISTRY 5 NOTES
Points to Remember
Students can record important points to remember from chapter 5 of NCERT Grade 12 Chemistry to do well in exams.
Adsorbent: Adsorbent on the surface.
Adsorbent: substance present in higher concentrations on the surface of the adsorbent. It is a type of adsorbent in which the adsorbent is held on the surface of the adsorbent by van der Waal forces.
Chemisorption is a type of adsorption in which the adsorbent is held on the surface of the adsorbent by chemical bonds.
Enthalpy of Adsorption: The enthalpy change associated with the adsorbent. It is always negative.
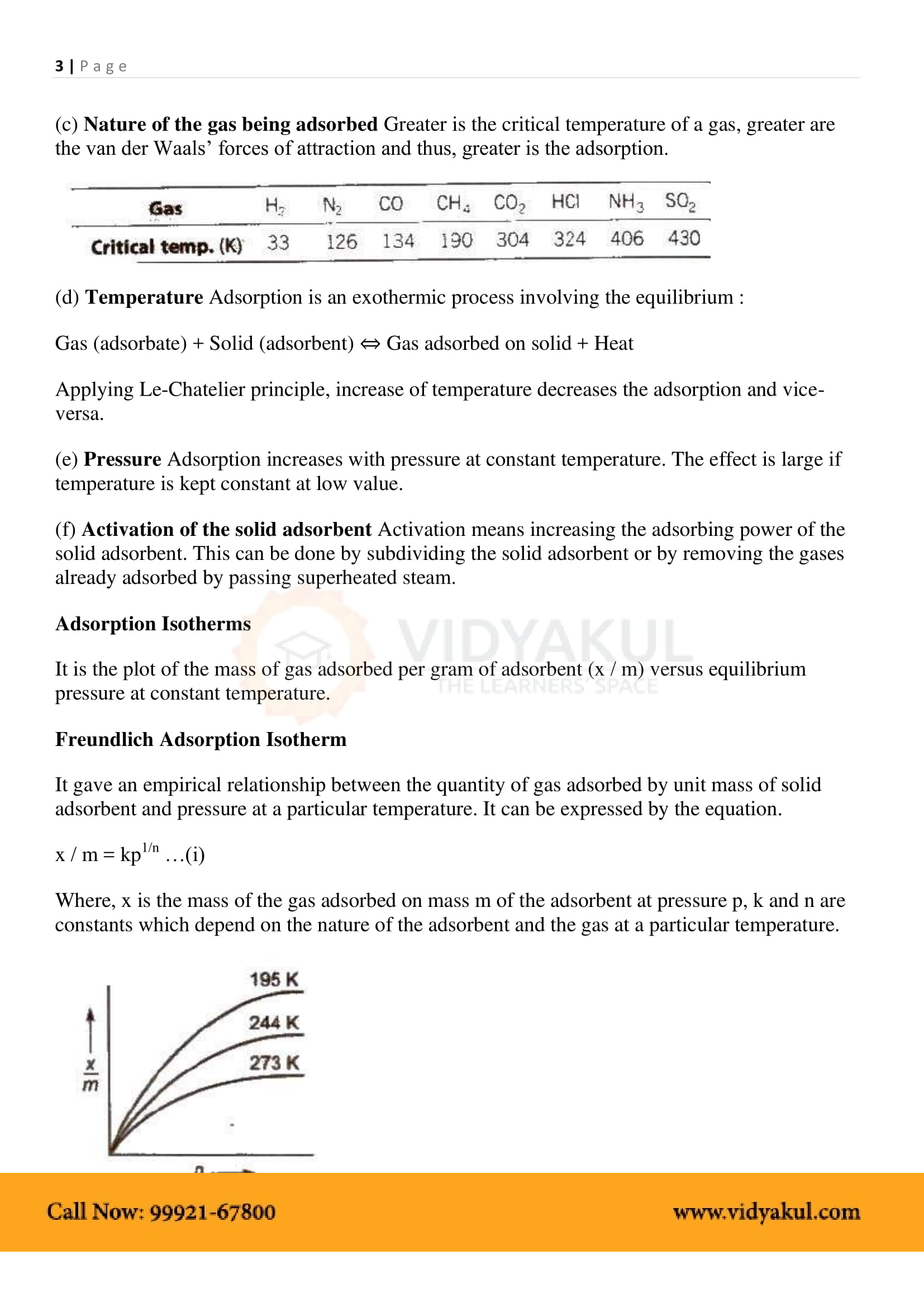
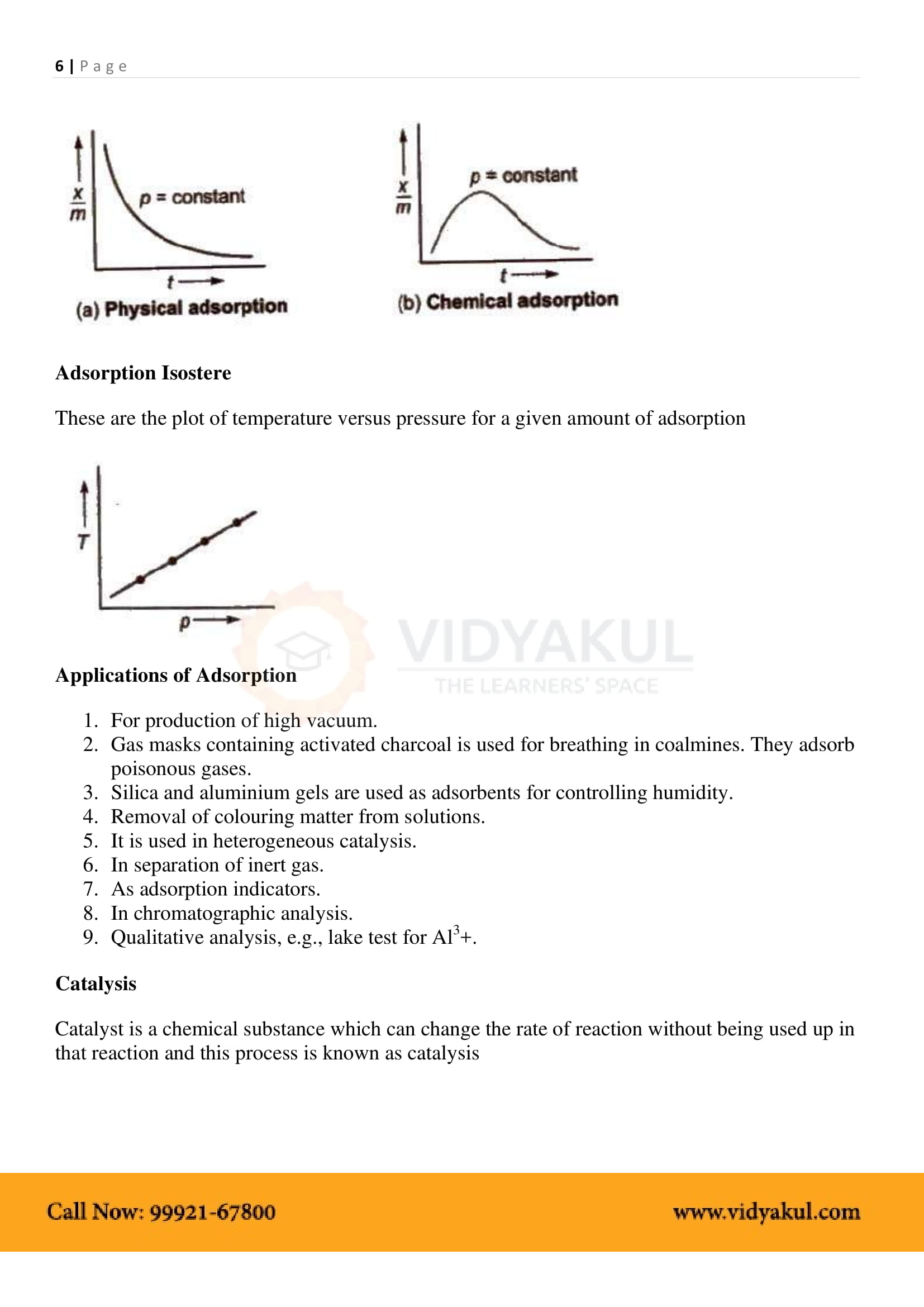
The degree of adsorption decreases with increasing temperature and increases with increasing pressure.
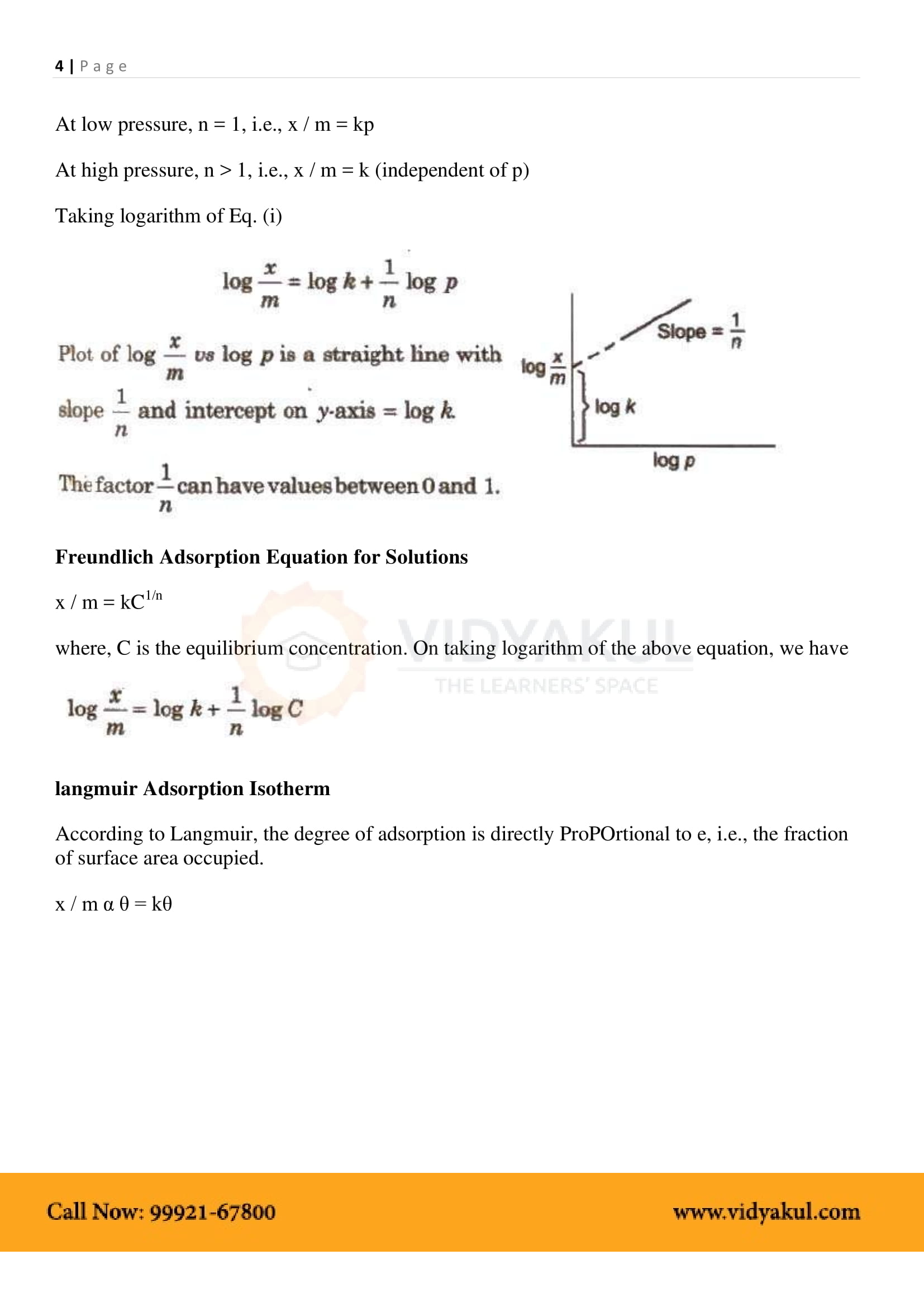
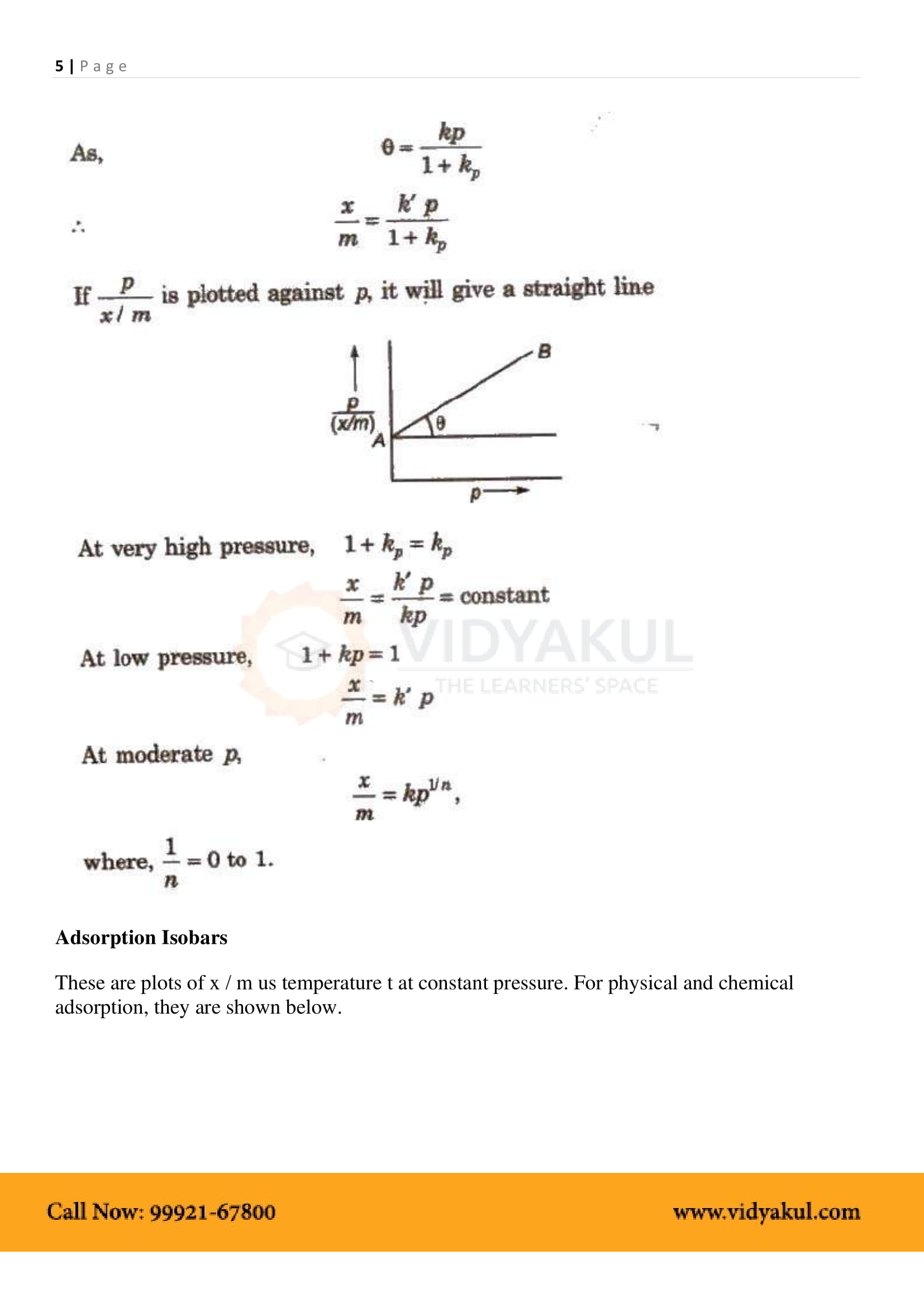
Isotherm adsorption: The relationship between the degree of adsorption and the pressure at a constant temperature.
Topics and Sub-Topics
Surface chemistry is one of the easiest subjects in Grade 12. While solving problems, students will become familiar with problem-solving methods and will be able to easily answer all types of questions posed. Concepts and notes will soon become interesting and students will develop speed and accuracy in problem-solving. Furthermore, students can practice the questions provided by Vidyakul for free and get good marks in the exams. Below is a list of Chemistry subjects for Grade 12 Chapter 5 Chemistry of Surfaces.
Download this solution for FREE Download This PDF
Download Vidyakul App for more Important videos, PDF's and Free video lectures.



Important Links:
Few Important Questions
What is the definition of a ‘Catalyst’?
Any substance that increases the rate of a reaction without itself being consumed is called a Catalyst.
What is ‘Chemical Kinetics’?
The branch of physical chemistry that is concerned with understanding the rates of chemical reactions.
What is the meaning of ‘Adsorption’?
Adsorption is the sticking of atoms or molecules to a surface. This surface is known as an Adsorbent.
Practice Questions
Explain the difference between absorption and adsorption with an example.
Define lyophobic and lyophilic sols with one example each. Also, explain which sol is easily coagulated and why?
Explain how the emulsion is stabilized with emulsifiers. Name any two emulsifiers.
What is aerosol? Write one example
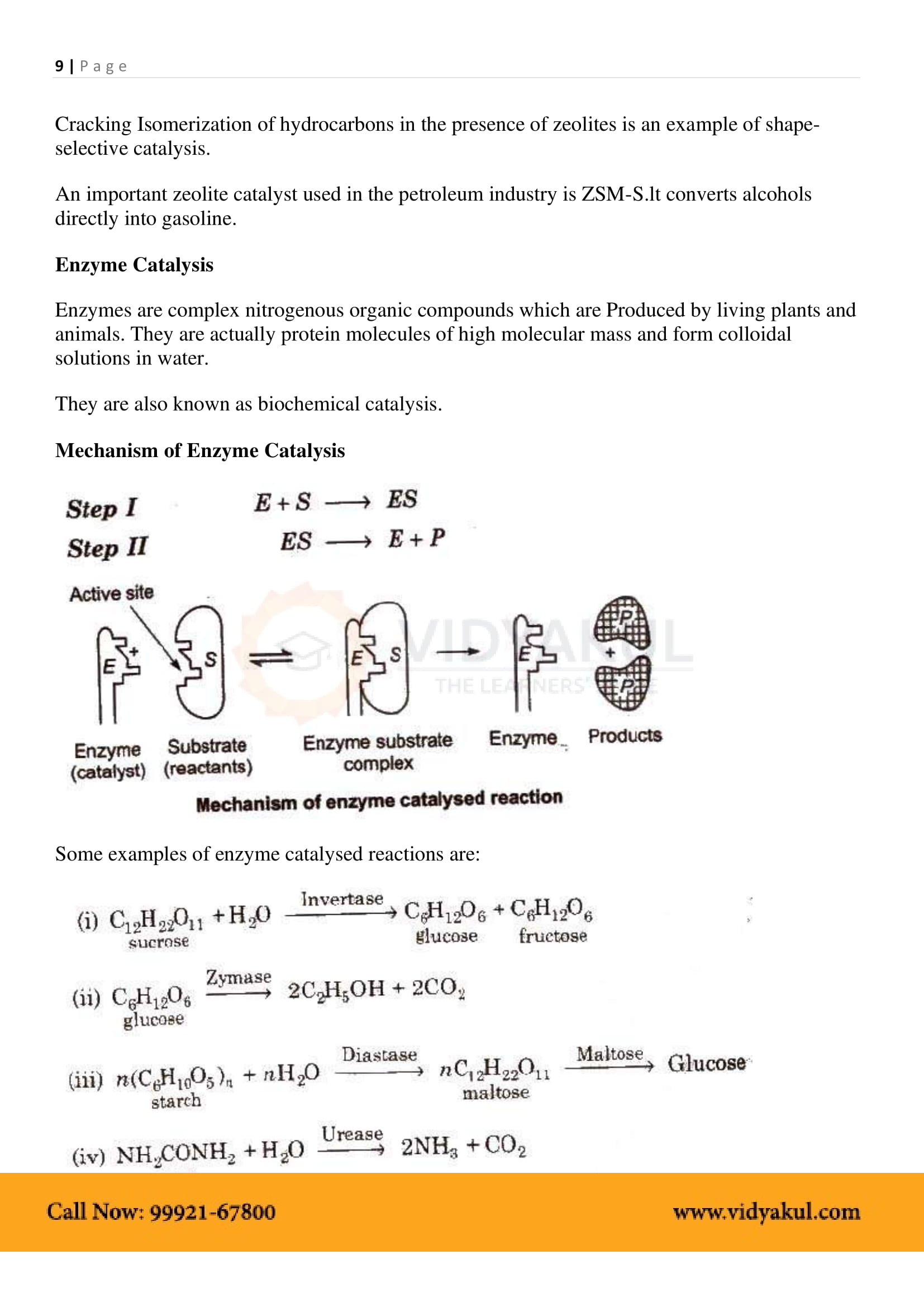
Define enzymes and explain enzyme catalysis mechanism.



