Polymers Class 12 Notes

Class 12 Chemistry Chapter 15 Polymers Notes- PDF DOWNLOAD
Chapter 15 Polymers
Experienced Vidyakul tutors have prepared NCERT solutions for Class 12 Chemistry Chapter 15. Polymers are large macromolecules produced by the polymerization of monomers. Students learn about the different types of polymers and types of polymerization processes. NCERT Chemistry notes for Grade 12 Chapter 15 contains answers to your polymer textbook and sample questions.
CBSE CLASS 12th CHEMISTRY 15 NOTES
Points to Remember
Some of the important points to remember from the NCERT Solutions for Class 12 Chemistry Chapter 15 are as follows:
The word ‘polymer’ is coined from two Greek words; ‘poly’ means many and ‘mer’ means unit or part.
The term polymer is defined as huge molecules having high molecular mass.
These are also called macromolecules, formed by joining repeating structural units on a large scale.
Therefore, the various applications depend upon their unique mechanical properties like tensile strength, elasticity, etc.
These mechanical properties are governed by intermolecular forces, e.g., Van Der Waals forces and hydrogen bonds present in the polymer.
The forces also bind the polymer chains.
Therefore, polymers are classified into four sub-groups based on the magnitude of their intermolecular forces.
Topics and Sub- topics
Professional teachers have addressed Chapter 15 “NCERT notes for Grade 12 in accordance with the latest CBSE guidelines. Year 12 students can be under pressure to do well on exams. To get good grades, students should use the NCERT notes. The Vidyakul NCERT notes are free and take all the doubts out of your head.
Students can use Vidyakul at any time and have free access to its content. Here is a list of important topics from Polymers.
Download this solution for FREE Download This PDF






Important Links:
Few Important Questions
What are the different types of ‘Polymers’?
Polymers can be classified into four main categories: thermoplastics, thermosets, elastomers, and synthetic fibers
What are the different types of ‘Natural Polymers’?
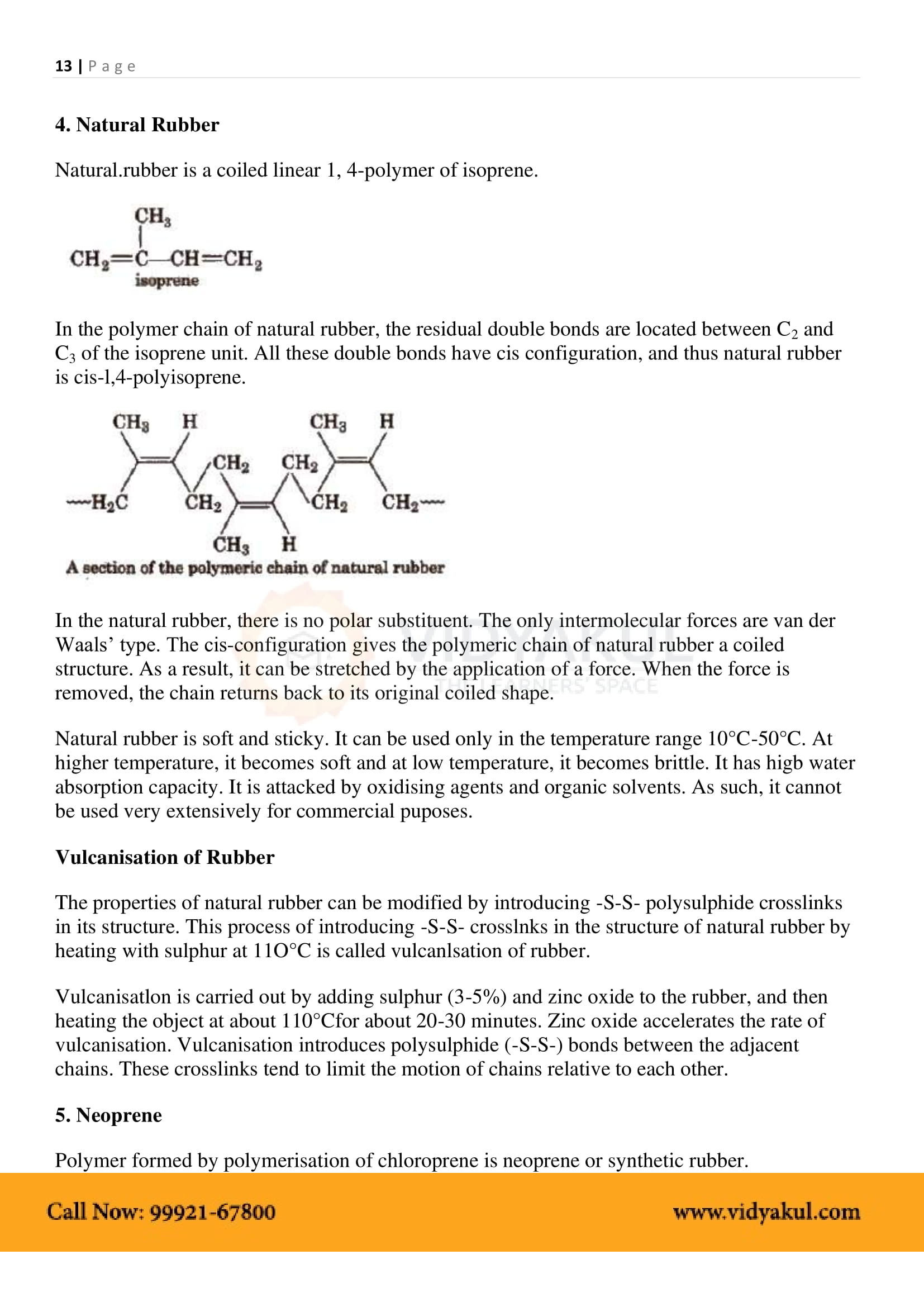
Some naturally occurring polymers are silk, wool, DNA, cellulose, and proteins.
What is the definition of ‘Polymerization reaction’?
Any process in which relatively small molecules, called monomers, combine chemically to produce a very large chainlike or network molecule is called a polymer. This process is called ‘Polymerization’.
Practice Questions
Distinguish between condensation polymerization and addition polymerization.
Write the monomeric repeating units for Nylon-6,6.
Define and give two examples of synthetic polymers and natural polymers.
Explain the process of obtaining dacron from ethylene glycol.
What is copolymerization? Give an example
Write the difference between thermosetting polymers and thermoplastics with examples.
What are Biodegradable Polymers?
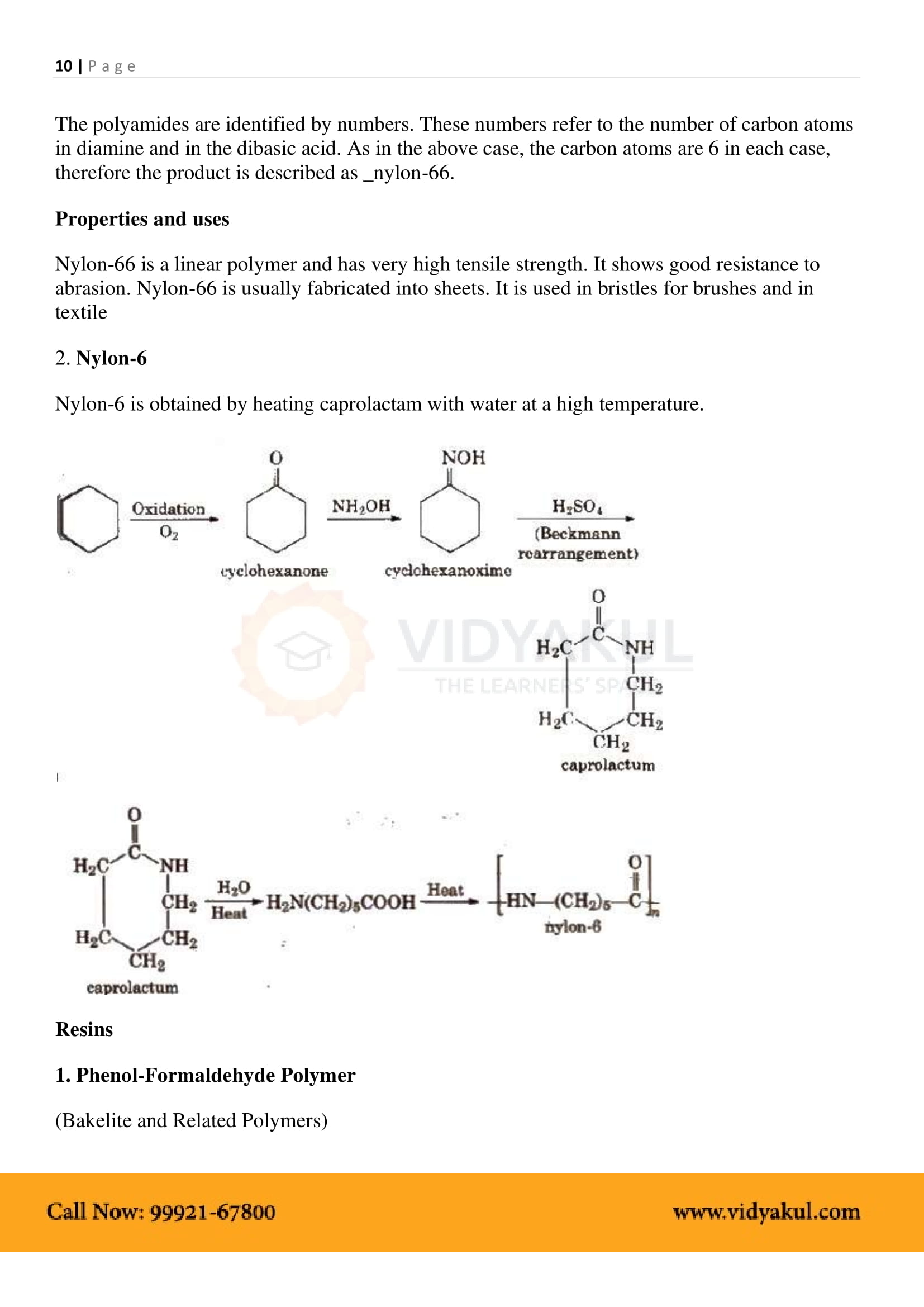
In “nylon 6, 6“, what does the designation “6, 6” mean?
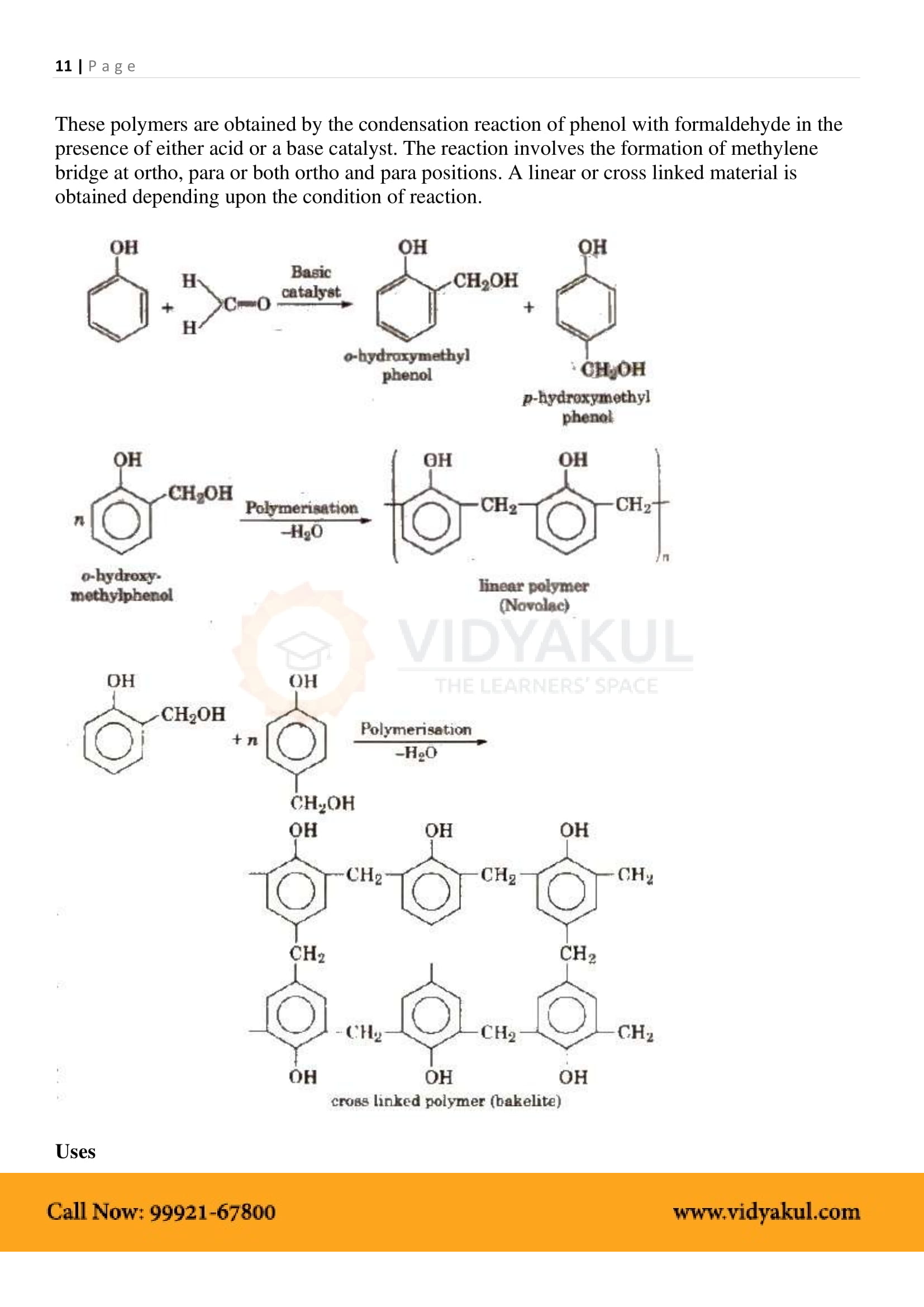
Mention two important uses of Bakelite?
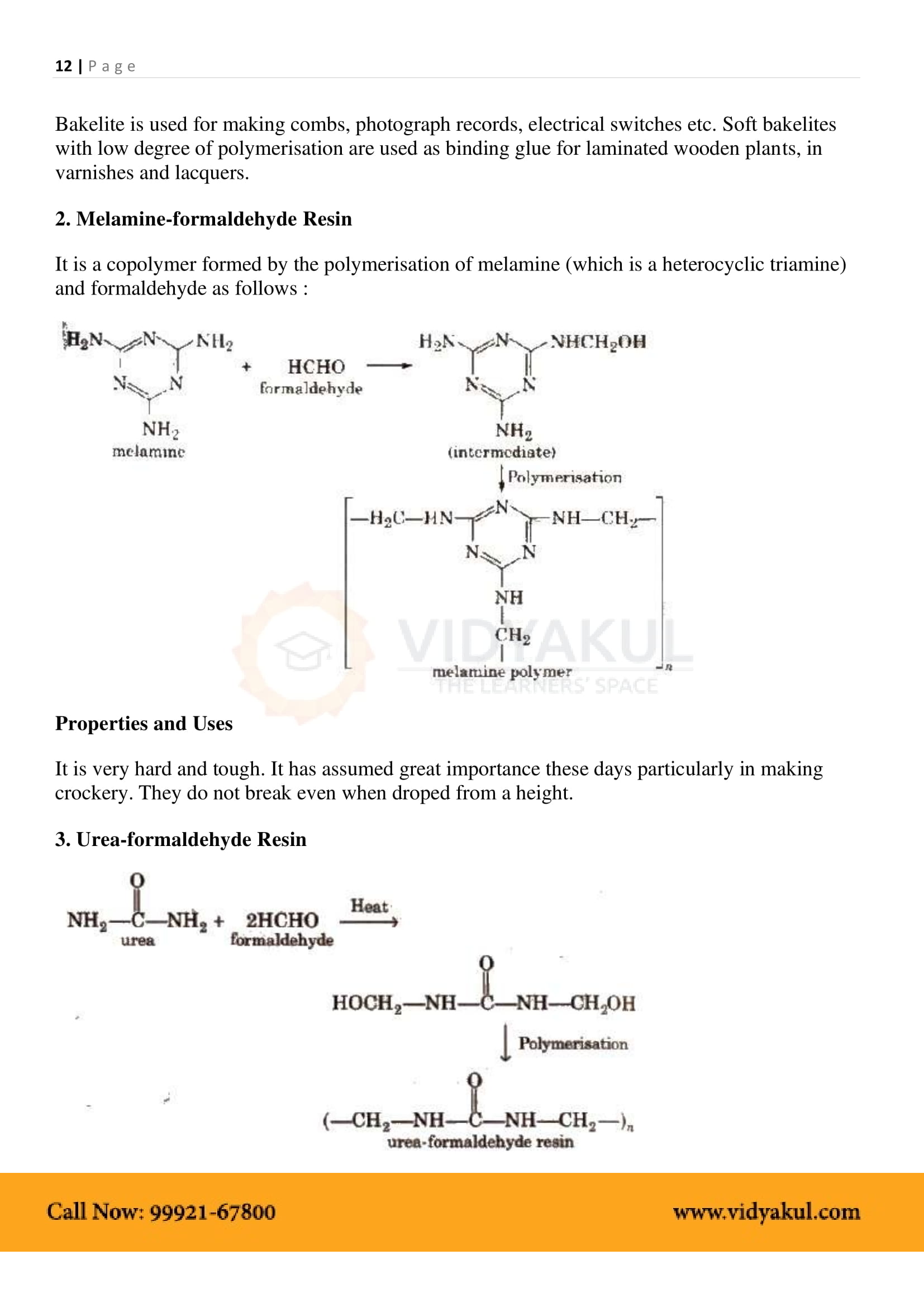
Name the polymers used in laminated sheets and give the name of monomeric units involved in its formation.



