Organic Compounds Containing Nitrogen Class 12 Notes

Class 12 Chemistry Chapter 13 Organic Compounds Containing Nitrogen Notes- Pdf Download
Chapter 13 Organic Compounds Containing Nitrogen
The NCERT notes for Grade 12 Chemistry Chapter 13 deals with amines. These notes help students understand the concept of amine in a simple way. It also helps students remember complex structural formulas. Students should seriously prepare for this chapter as it is also very important for many other competitions.
This chapter covers the structure of amines, their classification, nomenclature, preparation of amines, physical properties, chemical reactions and much more. Scroll down to access NCERT notes for Chapter 12 Grade 13 Chemistry as well as Important Topics.
CBSE CLASS 12th CHEMISTRY 13 NOTES
Points to Remember
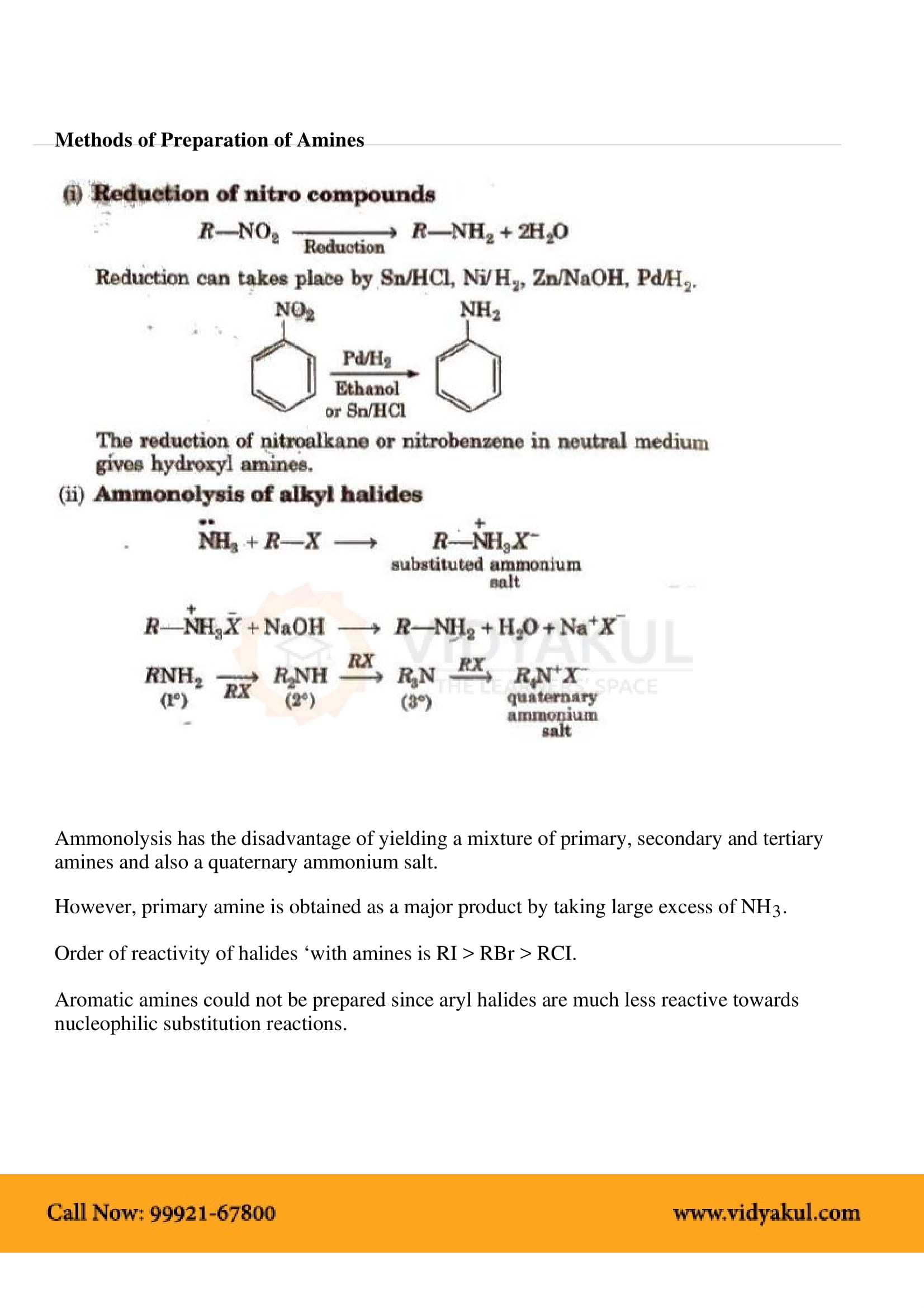
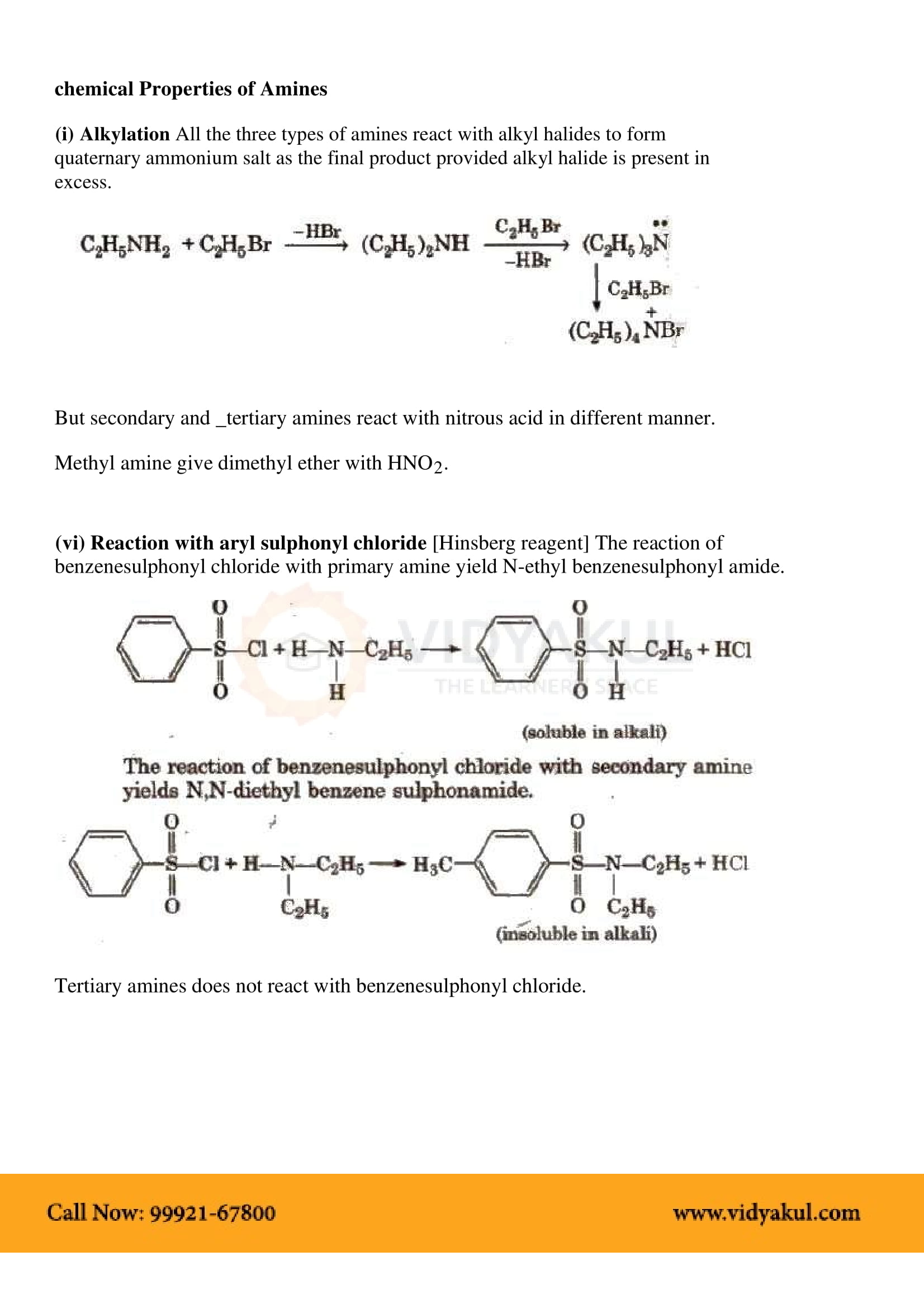
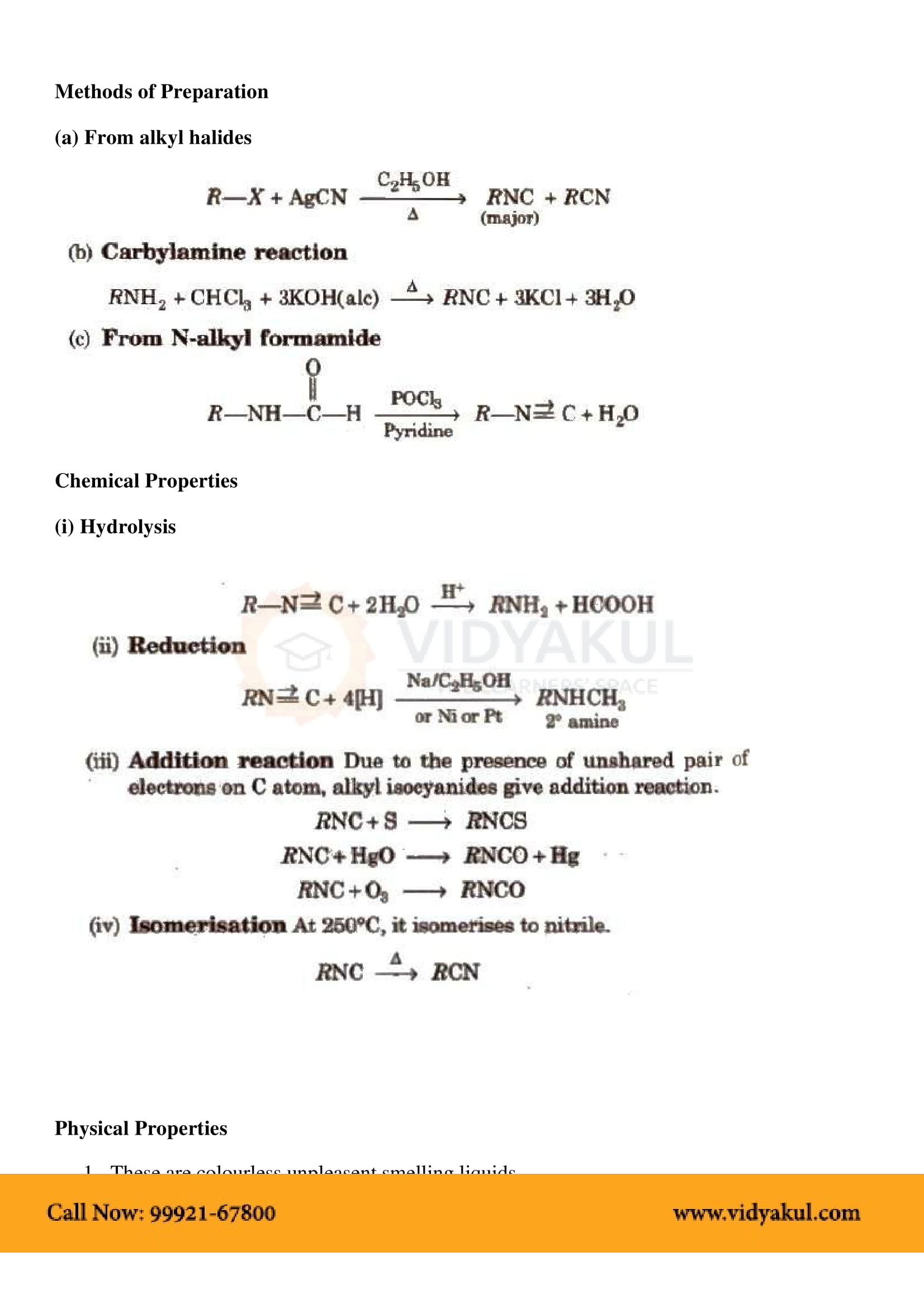
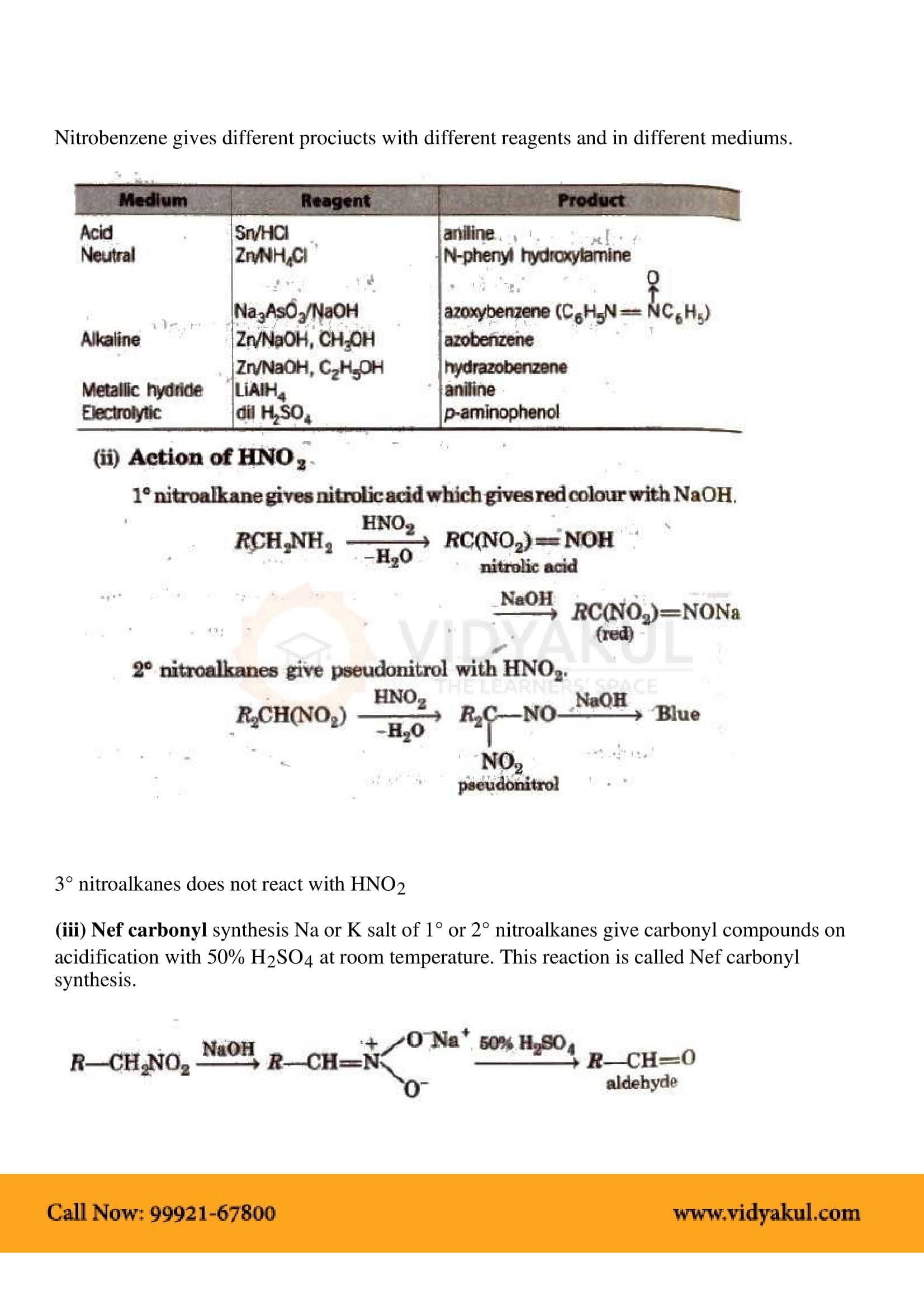
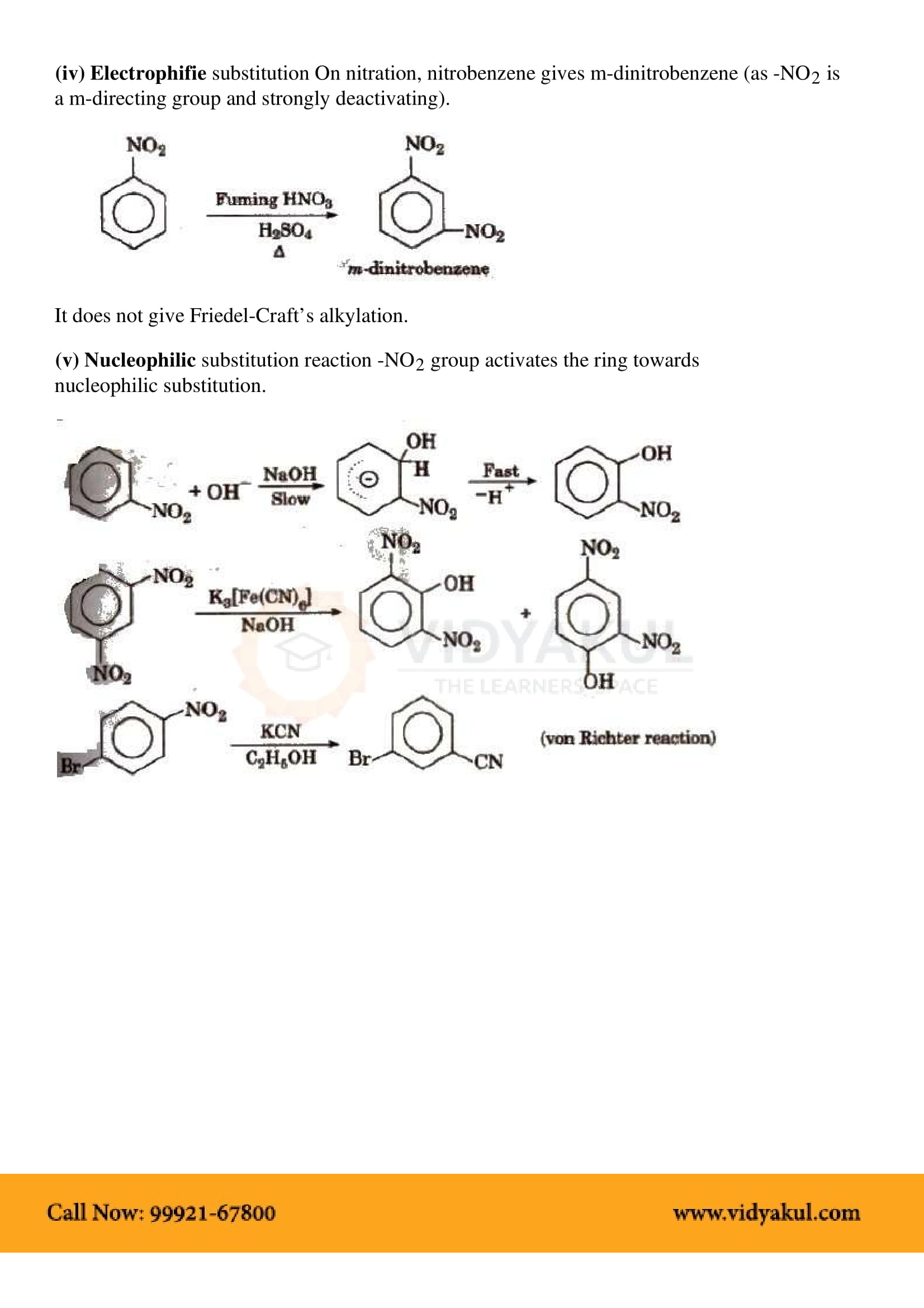
This chapter highlights the importance of amines that are derivatives of ammonia having a pyramidal structure. Students will learn about several methods of preparing amines and their chemical properties. Here are some important points for students to remember:
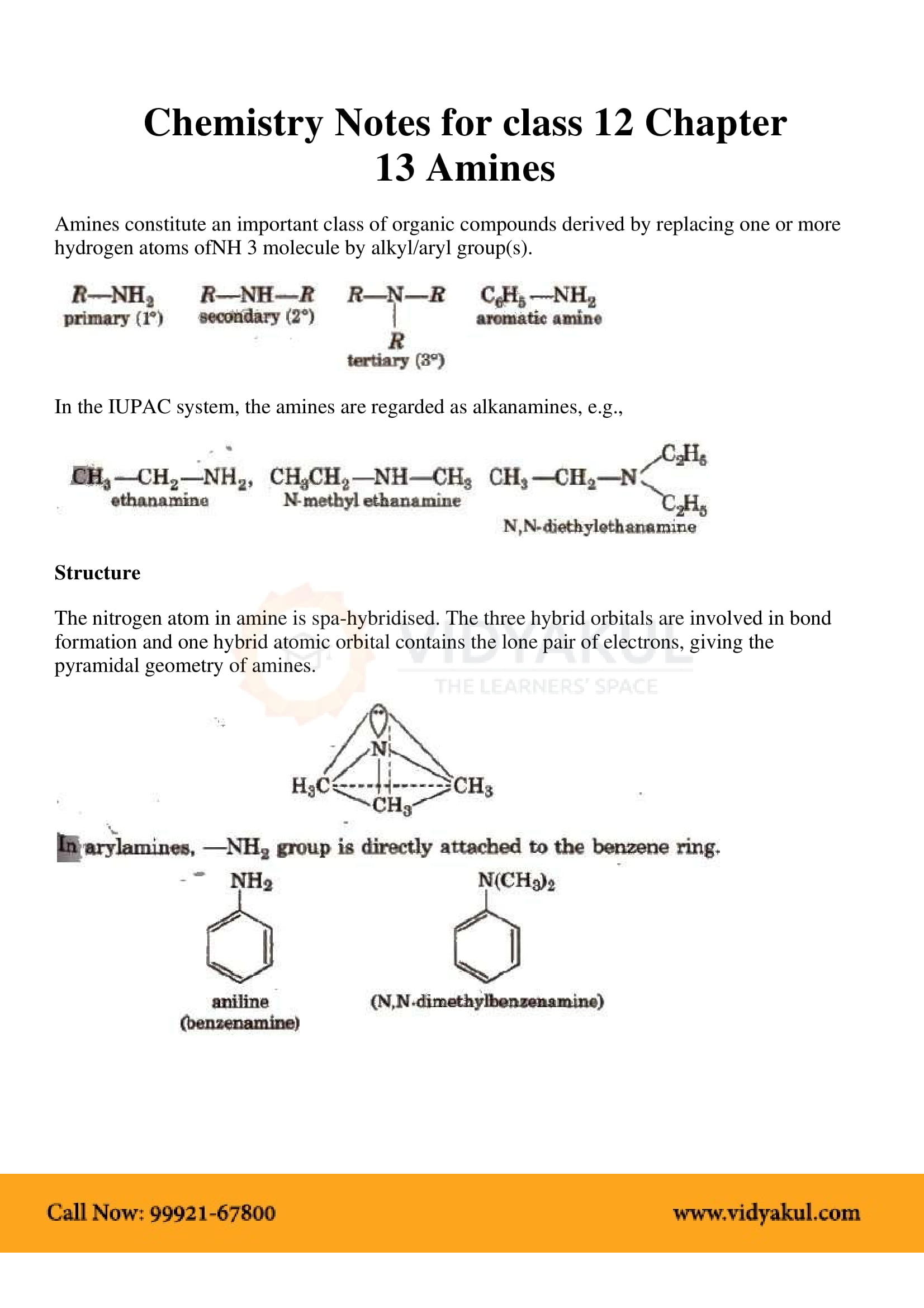
Amines can be considered as derivatives of ammonia, obtained by replacement of one, two or all the three hydrogen atoms by alkyl and/or aryl groups
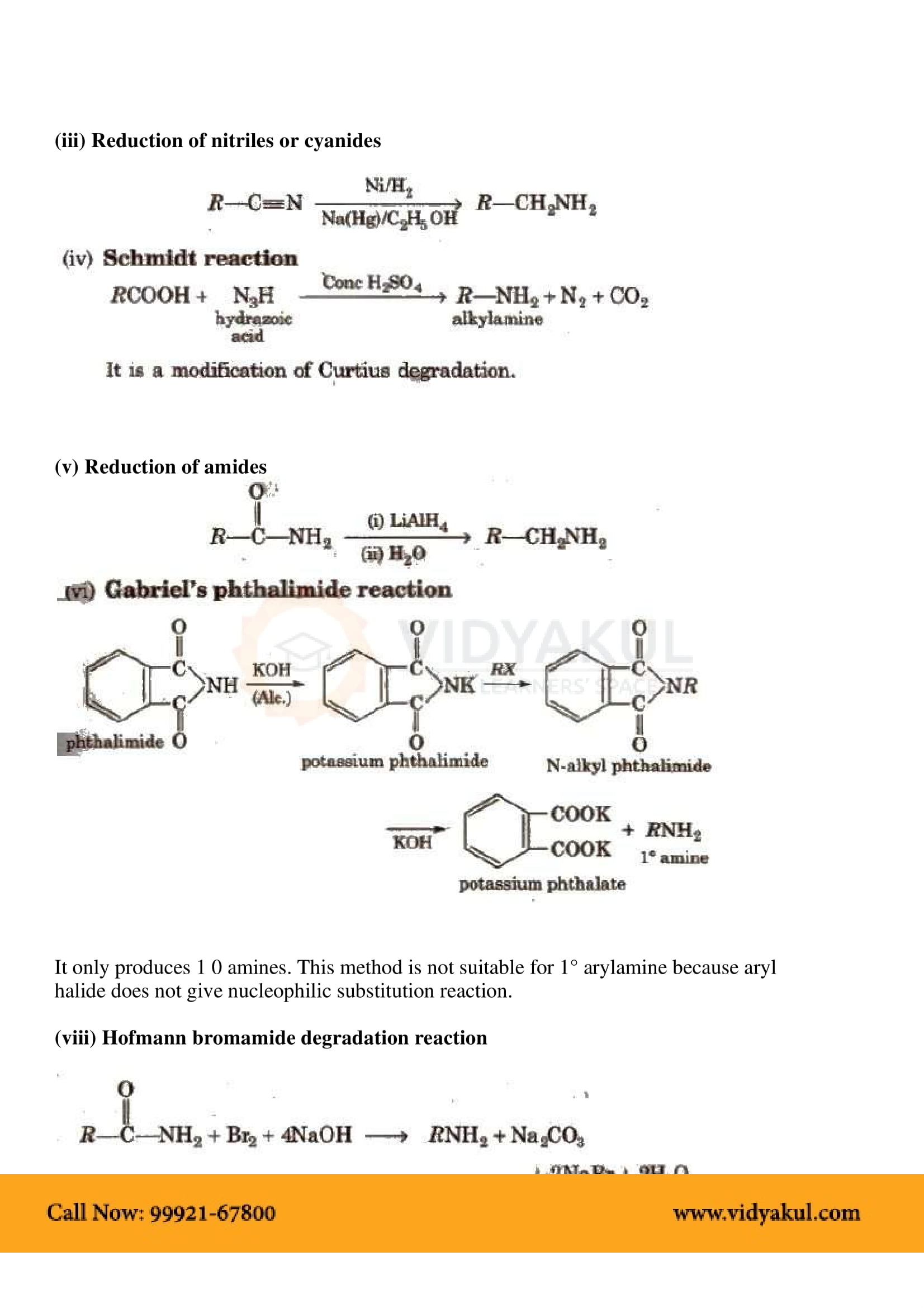
Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine.
The order of boiling points of isomeric amines is primary > secondary > tertiary.
Because of the presence of a lone pair of electrons on the nitrogen atom of -N˙H˙2 group, amines behave as Lewis bases.
All aliphatic amines are more basic than ammonia. In aqueous solution, the order of basic character is (CH3)2NH>CH3NH2>(CH3)3N
The free amine can be obtained from the ammonium salt by treatment with a strong base.
Topics and Sub- topics
Students may find it a bit difficult to understand the problems, but as they work through the problems, it becomes easier to master the topic and score well on the final exam. At Vidyakul, we provide notes to Chapter 13 Chemistry problems based on an updated outline. Moreover, all notes are provided by Vidyakul for free to get high scores in exams.
Students can find a list of important topics for chapter 13 as mentioned below:
Students can find the list of important topics of chapter 13 Amines as mentioned below:
Download this solution for FREE Download This PDF
Download Vidyakul App for more Important videos, PDF's and Free video lectures.





Important Links:
Few Important Questions
What are ‘Halides’?
Halides are chemical compounds that contain halogens. Halides are present in nature with some (salts and acids) being essential to human life.
What are ‘Amoratic amines’?
An aromatic amine is an organic compound consisting of an aromatic ring attached to an amine.
What is the common name of ‘Aniline’?
The common name of ‘Aniline’ is aminobenzene.
Practice Questions
Convert Ethanoic acid to methenamine and Propanoic acid to ethanoic acid
How to identify primary, secondary, and tertiary amines. Writes the reactions involved.
Is it possible to prepare aromatic primary amines by Gabriel phthalimide synthesis? Why?
Which of the two has a higher boiling point primary amines or tertiary amines? Why?
Which among the following is a strong base-
Aliphatic amines or aromatic amines? Explain with a valid reason.
Explain Hofmann’s bromamide reaction.
Distinguish between Methylamine and dimethylamine with a chemical test.



