Structure of The Atom Class 9 Notes
Chapter 4 Structure of The Atom
Science is a core subject in Year 9, so students are expected to study each chapter and topic thoroughly. CBSE Class 9 Science Chapter 4 is about the Structure of Atoms. In this chapter, students learn about the charged particles of matter, the structure of atoms, the distribution of electrons in different orbitals, valency, atomic and mass numbers, and isotopes. difficult. We provide about 2500 exercises. The structure of the atom is an important chapter for students as it helps students build a foundation of concepts based on it in high school. Scroll down to learn more.
Vidyakul provides over 3500 questions with 10 guides. Students learn different ways to solve specific problems and develop necessary presentation skills. Class 9 NCERT notes for carbon and compound fields are free.
Scroll down to see all information related to NCERT Class 9 science.
CBSE CLASS 9th CH-4
Points to Remember
While studying, the important points to remember from CBSE Class 9 Science Chapter 4 are as follows:
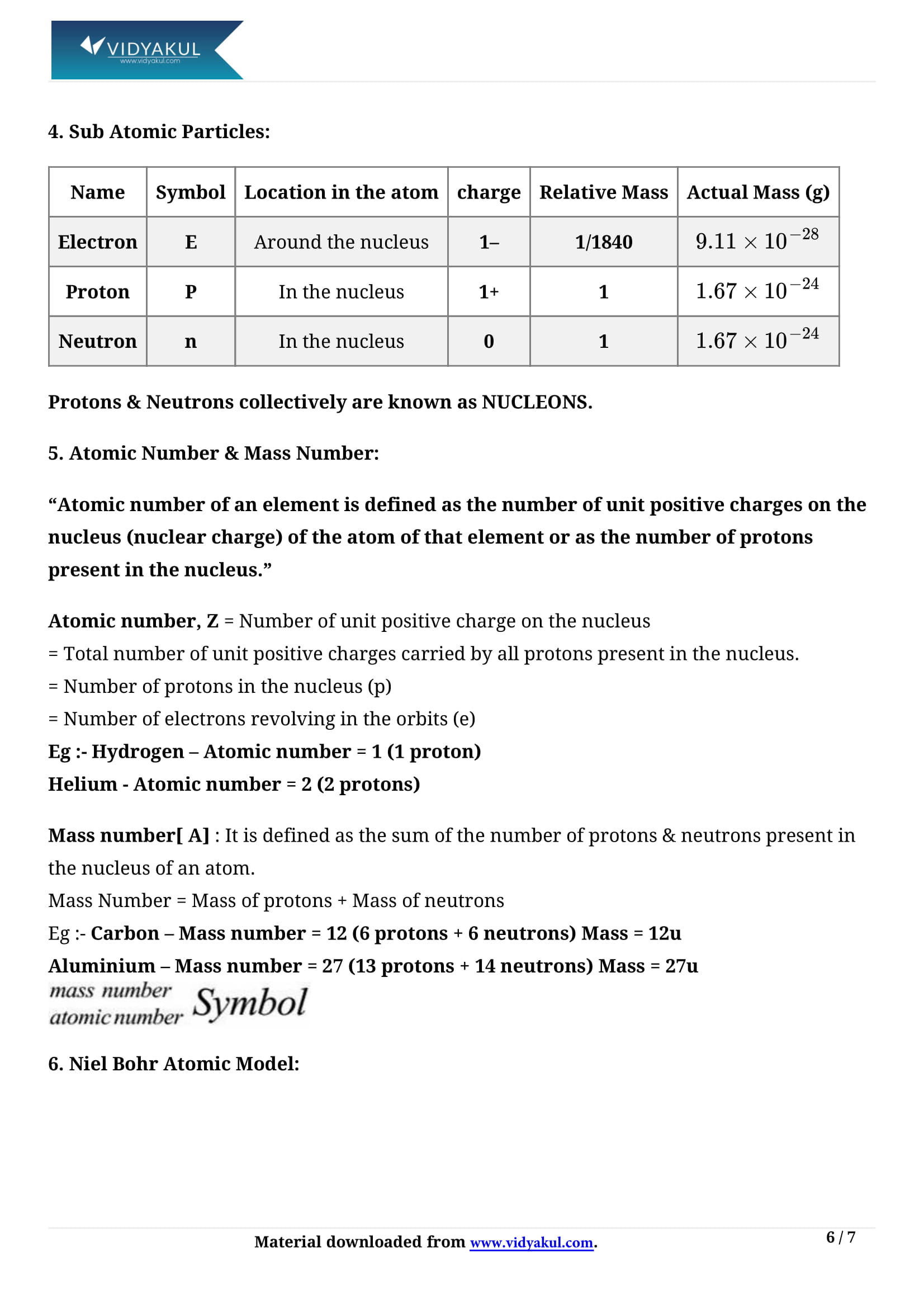
Goldstein discovered the proton, whereas J.J. Thomson discovered electrons.
Valency is defined as an atom’s total capacity.
Because of their stable electronic configuration, inert gases do not participate in chemical bonding.
An element’s atomic number is equal to the number of electrons or protons in the neutral atom.
Isotopes are atoms with different mass numbers of the same element.
Isobars are atoms with the same mass number but different atomic numbers from different elements.
Elements are distinguished by the number of protons or electrons they contain.
Topics and Sub-topics
In this chapter, students learn basic atomic terms such as atomic number, atomic mass, neutrons, and valency. You will also learn about different atomic models, functions and limitations. The study begins with the basic structure of matter's charged particles and atoms.
Chapter 4 of Science Class 9 deals with the discovery and structure of the atom. Students will also be exposed to the work of renowned scientists who have developed various models and theories. Before delving deeper into the NCERT crystal structure of class 9 atoms, let's review the topics and subtopics in this chapter:
Few Important Questions
What are the features of ‘Thomas atomic model’?
J.J. Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom.
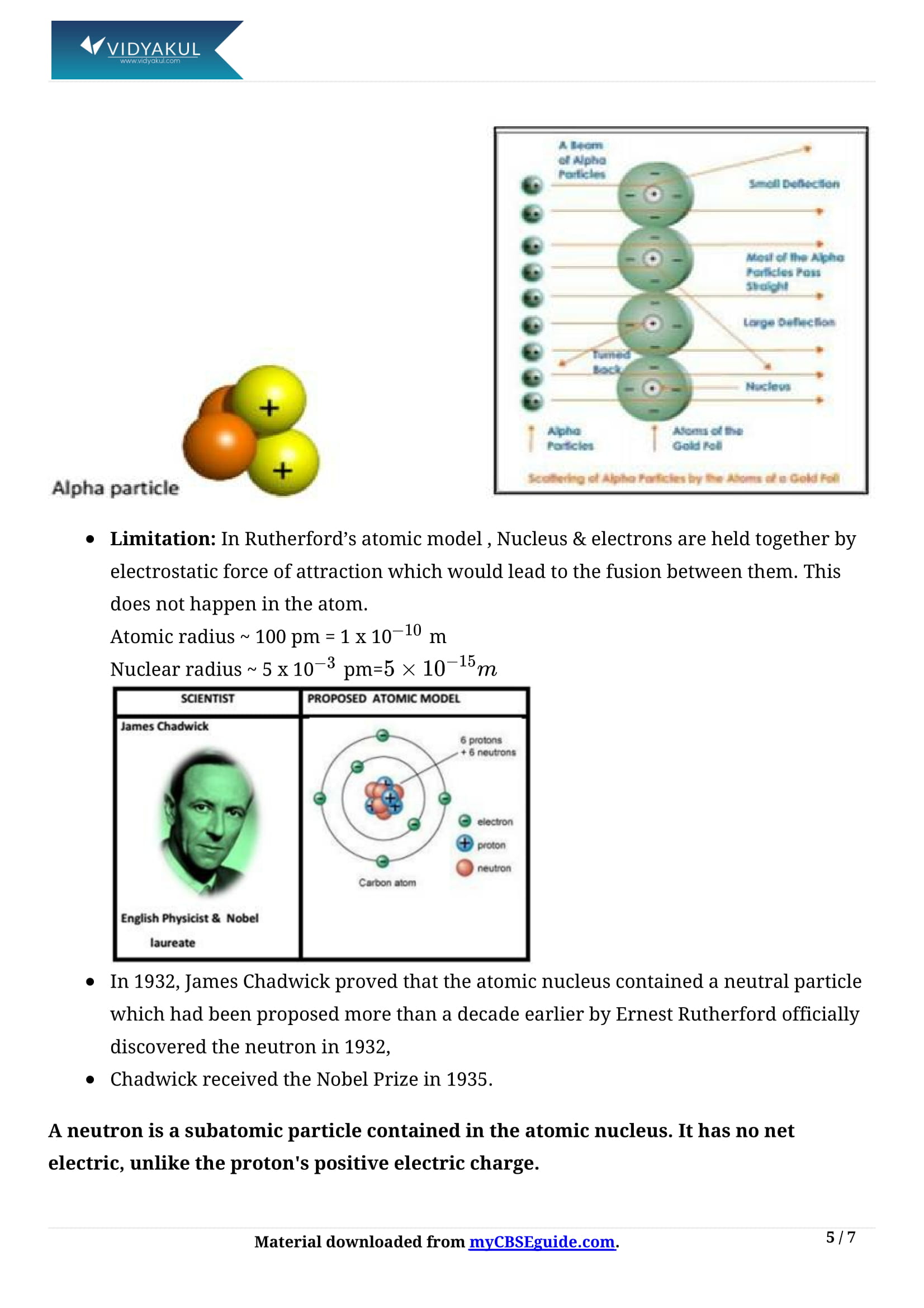
Who was ‘Ernest Rutherford’?
Ernest Rutherford was a New Zealand physicist who came to be known as the father of nuclear physics.
What did ‘Neils Bohr’ discover?
Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values.
Goldstein discovered the proton, whereas J.J. Thomson discovered electrons.
Valency is defined as an atom’s total capacity.
Because of their stable electronic configuration, inert gases do not participate in chemical bonding.
An element’s atomic number is equal to the number of electrons or protons in the neutral atom.
Isotopes are atoms with different mass numbers of the same element.
Isobars are atoms with the same mass number but different atomic numbers from different elements.
Elements are distinguished by the number of protons or electrons they contain.
What are the features of ‘Thomas atomic model’?
Who was ‘Ernest Rutherford’?
What did ‘Neils Bohr’ discover?
Learn more about it in Structure of the Atom Class 9 Notes pdf.
Download this solution for FREE Download this PDF