Atoms and Molecules Class 9 Notes

Chapter 3 Atoms and Molecules
This chapter covers all the basics of atoms and molecules. This chapter is very important as it requires a basic understanding of atoms and molecules to understand the dependent and complex topics of classes X, XI and XII. This topic is also important when it comes to career prospects in chemistry and biotechnology. Students are advised to study this chapter carefully and clear all major doubts. The chapter "Atoms and Molecules" mainly explains the Law of Conservation of Mass, the Law of Specific Proportions, Postulates of Dalton's Atomic Theory, Atoms, Molecules, and Ions.
Vidyakul provides over 1,000 exercises from Chapter 3 Science for 9th grade to help students understand the subject. These exercises from Vidyakul are free and expand students' knowledge. To get good grades, students must study all text problems designed by Vidyakul. Vidyakul learning platform simplifies exam preparation.
Continue reading this article for more details.
CBSE CLASS 9th CH-3
Points to Remember
We have provided some of the important points to remember for NCERT Class 9 Science chapter 3 as mentioned below:
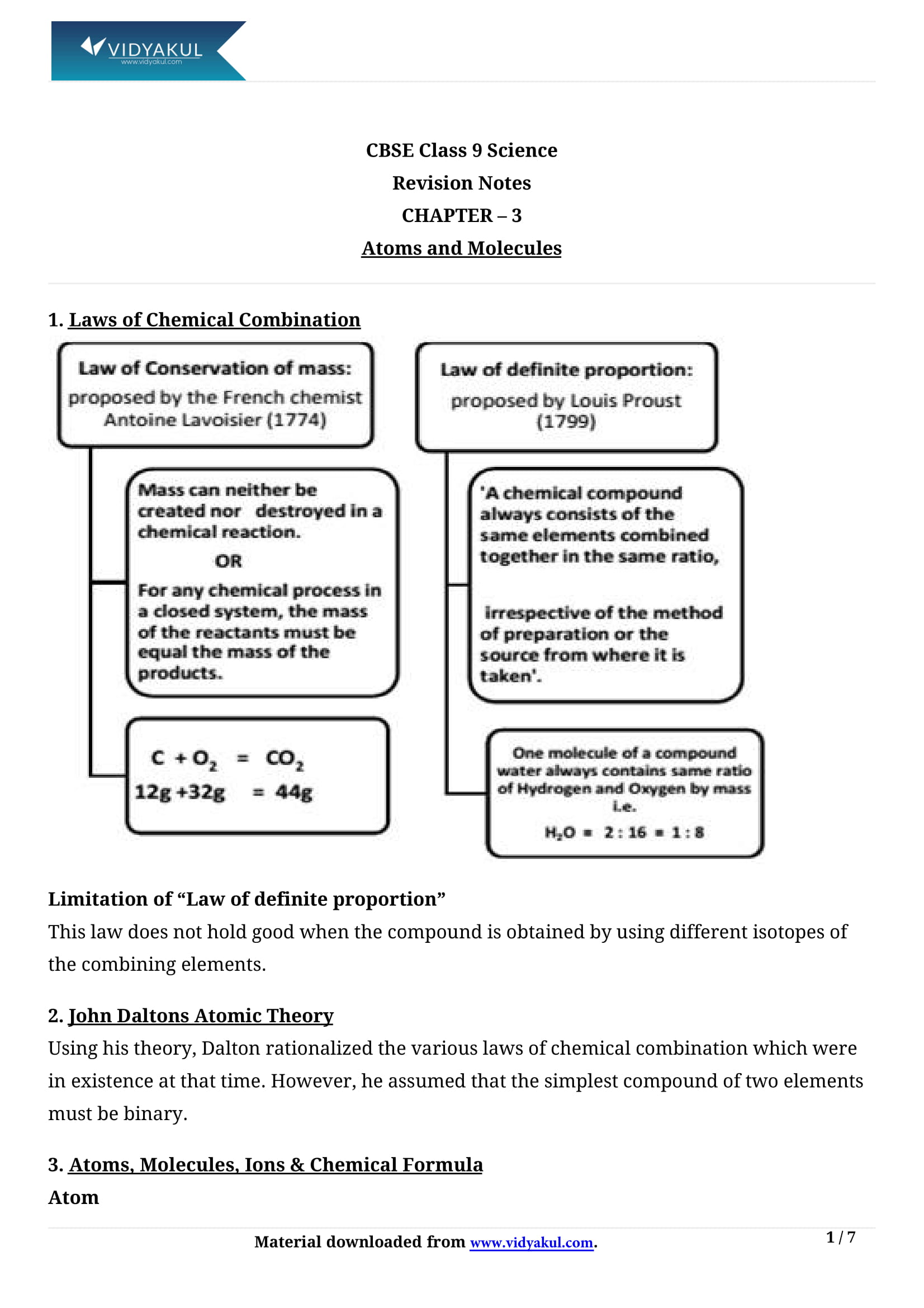
Laws of chemical combination:
(i)The combination between the elements are governed by laws of chemical combination.
(ii) The law of conservation of mass was given by Lavoisier.
(iii) The law of constant proportions was given by Louis Proust.
(iv) The law of constant proportions is applicable only to pure chemical compounds.
Atom:
(i) John Dalton was the first to state that atom is the smallest indivisible part of matter.
(ii) Atom is the smallest tiny particle of matter which can take part in chemical combination. It may or may not exist independently.
(iii) Dalton’s Atomic theory has certain limitations or defects.
(iv) Scanning tunneling microscope (STM) can be used to have a pictorial view of the atoms.
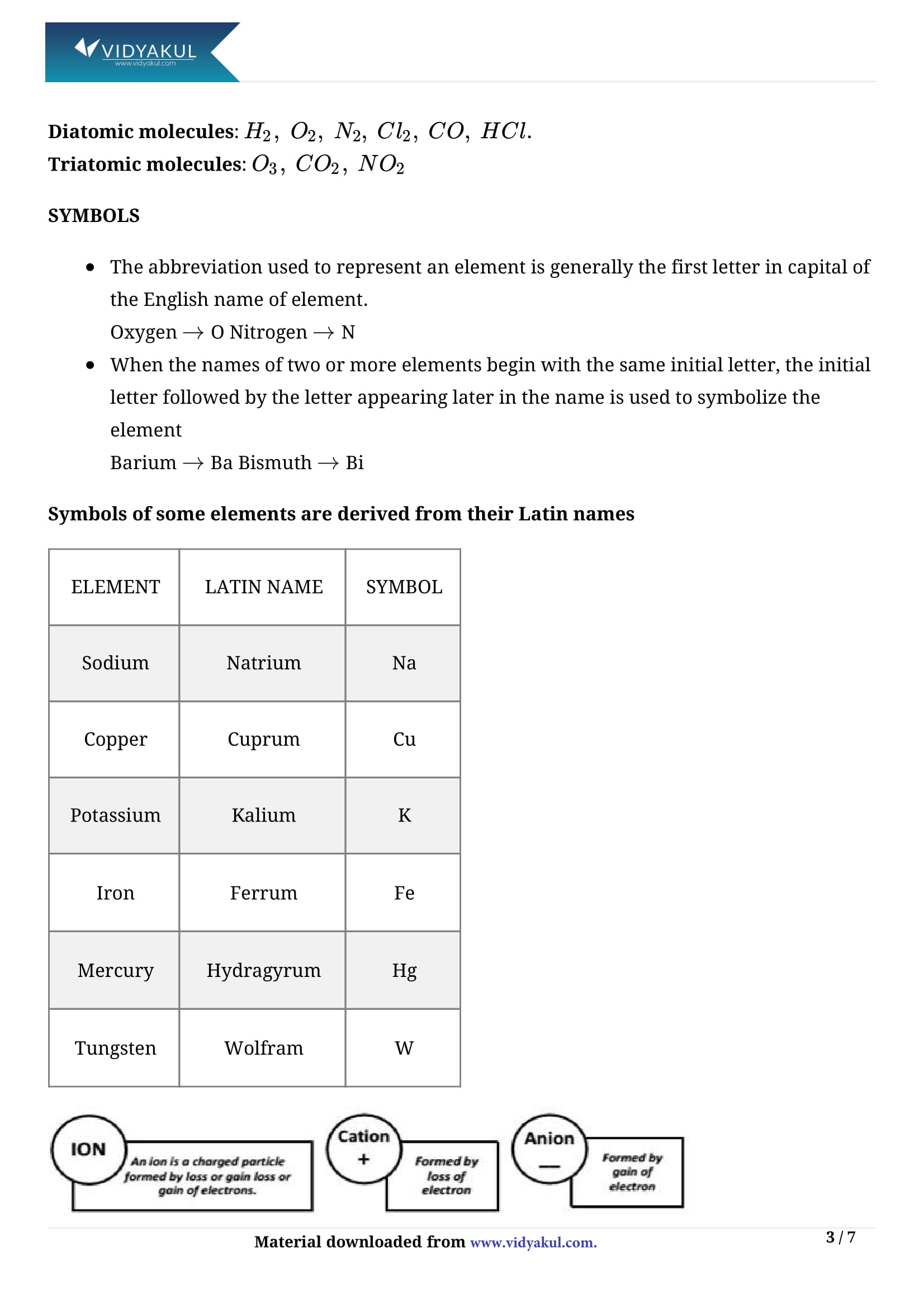
(v) Symbols of elements give the shorthand representation of the name of elements.
(vi) Chemical symbols for the elements were given by Berzelius.
(vii) No two elements can have the same symbol. Isotopes of an element have same symbol.
(viii) Atomic masses of the elements are relative masses and not their actual masses.
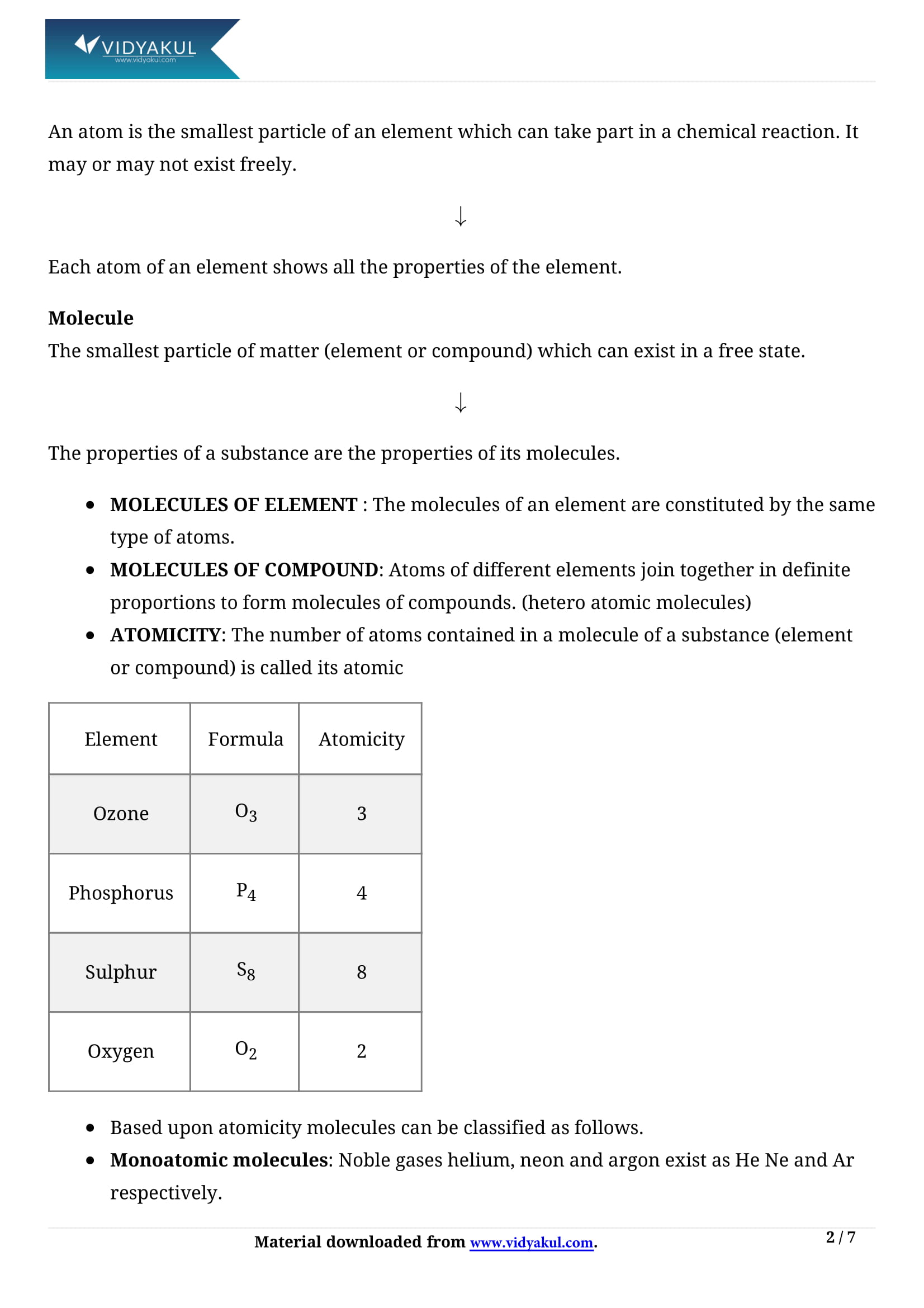
Molecule:
(i) In a molecule, the atoms of same or different elements are chemically bonded to each other.
(ii)The number of atoms present in one molecule of element represent its atomicity.
(iii) Noble gases are monoatomic.
(iv)An ion is an atom or group of atoms having positive or negative charge.
(v)A cation has positive charge whereas an anion has negative charge
(vi)Number of electrons and protons in a neutral atom is equal. Cations as well as anions of an atom do not have equal number of electrons and protons.
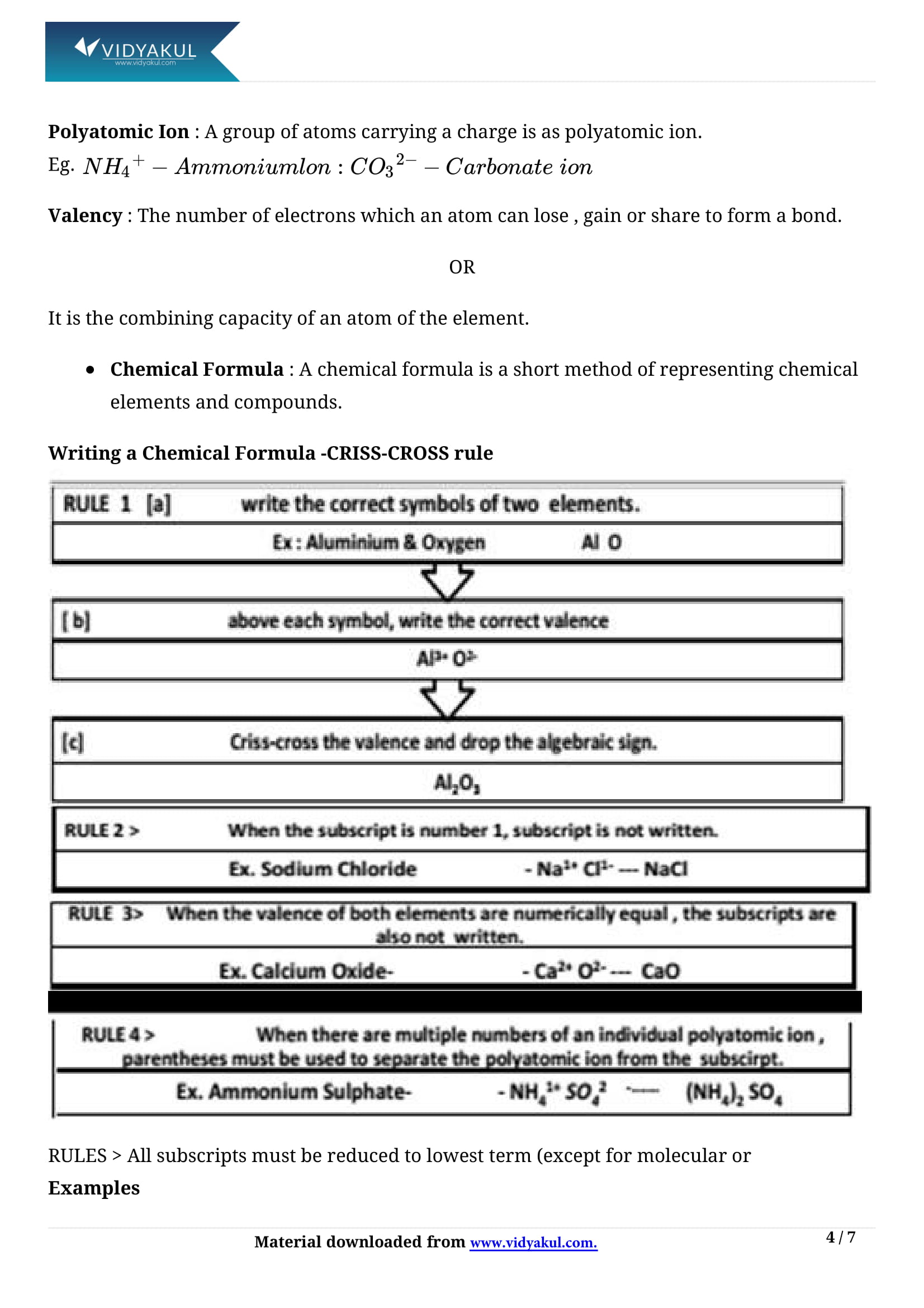
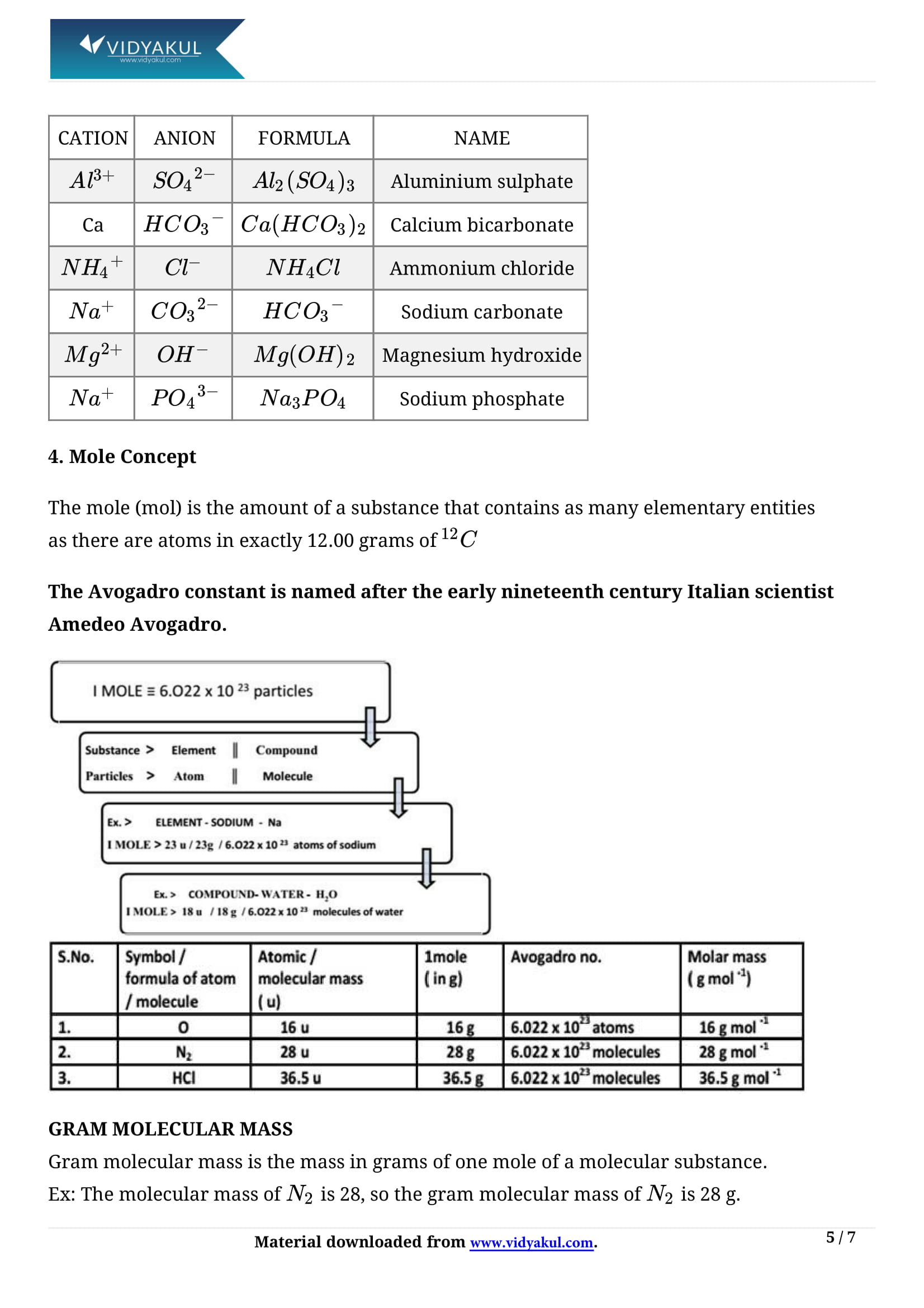
Writing chemical formula:
(i) Chemical formula is the representation of a molecule in terms of the symbols of the constituting atoms.
ii) Valency of an element is the combining capacity of its atoms
Topics and Sub-topics
Atoms and molecules are some of the easiest concepts to understand. Students are encouraged to go through the concepts first so that they can easily solve the problems. It is recommended to solve as many problems as possible to increase the speed and accuracy of problem solving. The more questions you practice, the easier it will be to answer final exam questions.
Vidyakul NCERT notes for Class 9 Science is the best learning resource for students. You can learn and practice Grade 9 science chapter concepts for free on Vidyakul. This chapter covers topics such as the chemical properties of metals and the physical properties of metals and nonmetals. Vidyakul also allows students to track and improve their progress.
Before you start preparing for NCERT Class 9 Science, you should be familiar with the topics and subtopics of NCERT Class 9 Science Chapter 3. A thorough knowledge of the subject will help you absorb information quickly.
Few Important Questions
What is difference between atoms and molecules?
Atoms refer to the mos basic or smallest unit of a chemical element. Whereas, molecules are composed of 2 or more atoms held together.
Who was Dalton?
John Dalton was an English chemist, physicist and he was best known for his Atomic Theory.
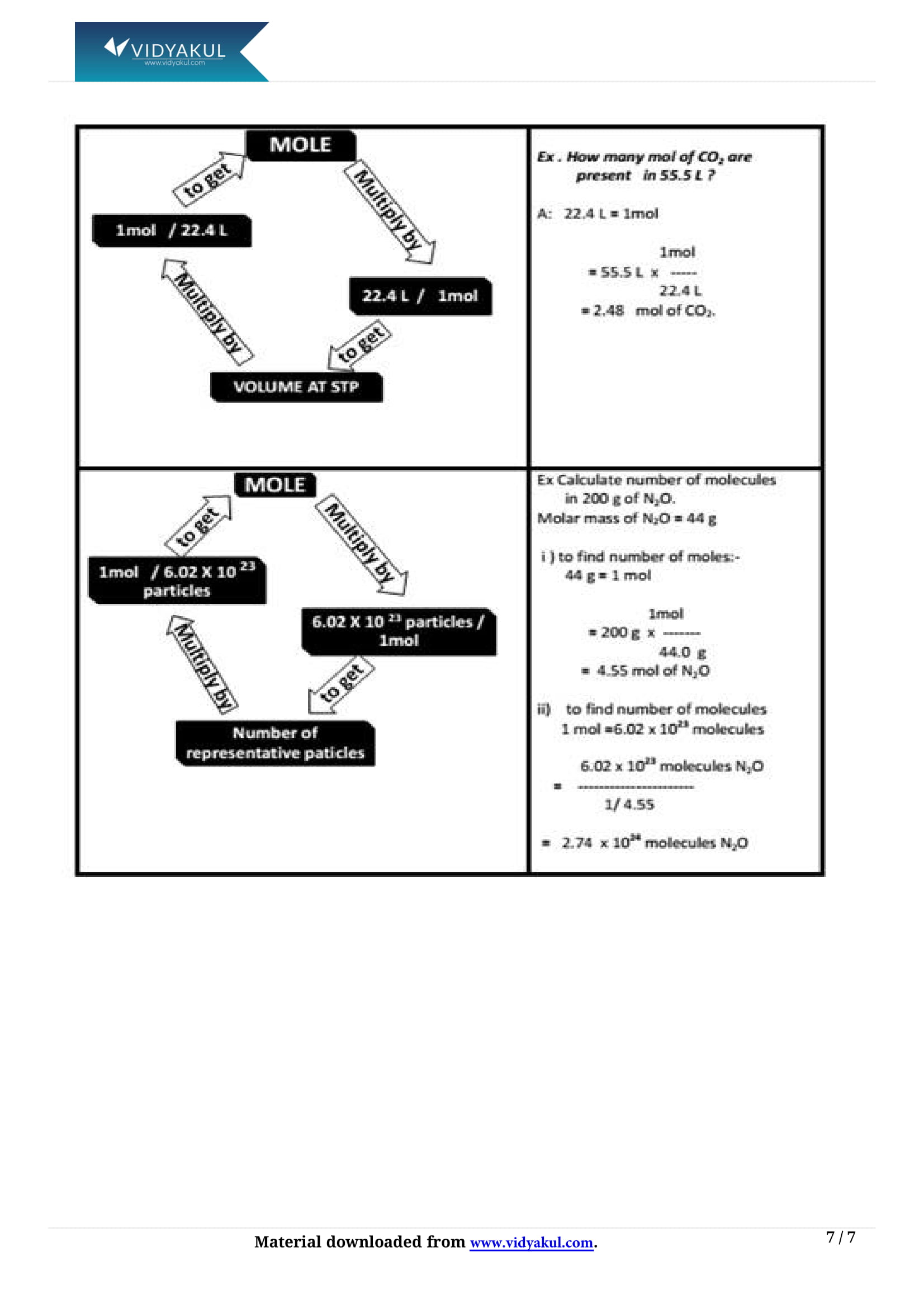
What is a mole?
Mole is a standard unit for measuring the amount of any substance.
Laws of chemical combination:
(i)The combination between the elements are governed by laws of chemical combination.
(ii) The law of conservation of mass was given by Lavoisier.
(iii) The law of constant proportions was given by Louis Proust.
(iv) The law of constant proportions is applicable only to pure chemical compounds.
Atom:
(i) John Dalton was the first to state that atom is the smallest indivisible part of matter.
(ii) Atom is the smallest tiny particle of matter which can take part in chemical combination. It may or may not exist independently.
(iii) Dalton’s Atomic theory has certain limitations or defects.
(iv) Scanning tunneling microscope (STM) can be used to have a pictorial view of the atoms.
(v) Symbols of elements give the shorthand representation of the name of elements.
(vi) Chemical symbols for the elements were given by Berzelius.
(vii) No two elements can have the same symbol. Isotopes of an element have same symbol.
(viii) Atomic masses of the elements are relative masses and not their actual masses.
Molecule:
(i) In a molecule, the atoms of same or different elements are chemically bonded to each other.
(ii)The number of atoms present in one molecule of element represent its atomicity.
(iii) Noble gases are monoatomic.
(iv)An ion is an atom or group of atoms having positive or negative charge.
(v)A cation has positive charge whereas an anion has negative charge
(vi)Number of electrons and protons in a neutral atom is equal. Cations as well as anions of an atom do not have equal number of electrons and protons.
Writing chemical formula:
(i) Chemical formula is the representation of a molecule in terms of the symbols of the constituting atoms.
ii) Valency of an element is the combining capacity of its atoms
What is difference between atoms and molecules?
Who was Dalton?
What is a mole?
Learn more about it in Atoms and Molecules Class 9 Notes pdf.
Download this solution for FREE Download this PDF